Page 137 - Color_Atlas_of_Physiology_5th_Ed._-_A._Despopoulos_2003
P. 137
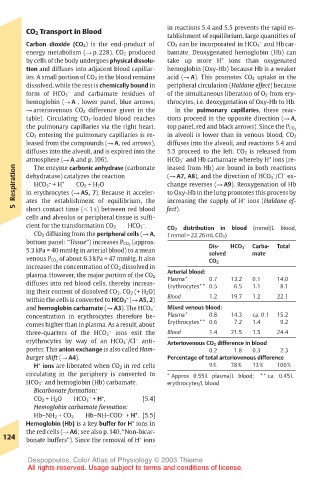
CO 2 Transport in Blood in reactions 5.4 and 5.5 prevents the rapid es-
tablishment of equilibrium, large quantities of
–
Carbon dioxide (CO 2) is the end-product of CO 2 can be incorporated in HCO 3 and Hb car-
energy metabolism (! p. 228). CO 2 produced bamate. Deoxygenated hemoglobin (Hb) can
by cells of the body undergoes physical dissolu- take up more H + ions than oxygenated
tion and diffuses into adjacent blood capillar- hemoglobin (Oxy-Hb) because Hb is a weaker
ies. A small portion of CO 2 in the blood remains acid (! A). This promotes CO 2 uptake in the
dissolved, while the rest is chemically bound in peripheral circulation (Haldane effect) because
–
form of HCO 3 and carbamate residues of of the simultaneous liberation of O 2 from ery-
hemoglobin (! A , lower panel, blue arrows; throcytes, i.e. deoxygenation of Oxy-Hb to Hb.
! arteriovenous CO 2 difference given in the In the pulmonary capillaries, these reac-
table). Circulating CO 2-loaded blood reaches tions proceed in the opposite direction (! A,
the pulmonary capillaries via the right heart. top panel, red and black arrows). Since the P CO 2
CO 2 entering the pulmonary capillaries is re- in alveoli is lower than in venous blood, CO 2
leased from the compounds (! A, red arrows), diffuses into the alveoli, and reactions 5.4 and
diffuses into the alveoli, and is expired into the 5.5 proceed to the left. CO 2 is released from
+
–
atmosphere (! A and p. 106). HCO 3 and Hb carbamate whereby H ions (re-
leased from Hb) are bound in both reactions
Respiration dehydratase) catalyzes the reaction (! A7, A8), and the direction of HCO 3 /Cl ex-
The enzyme carbonic anhydrase (carbonate
–
–
–
+
CO 2 + H 2O
HCO 3 + H
change reverses (! A9). Reoxygenation of Hb
to Oxy-Hb in the lung promotes this process by
in erythrocytes (! A5, 7). Because it acceler-
+
5 ates the establishment of equilibrium, the increasing the supply of H ions (Haldane ef-
short contact time (! 1 s) between red blood
fect).
cells and alveolus or peripheral tissue is suffi-
cient for the transformation CO 2 HCO 3 . – distribution in blood (mmol/L blood,
CO 2
CO 2 diffusing from the peripheral cells (! A, 1 mmol = 22.26 mL CO 2)
bottom panel: “Tissue”) increases P CO 2 (approx. Dis- – Carba- Total
5.3 kPa = 40 mmHg in arterial blood) to a mean solved HCO 3 mate
venous P CO 2 of about 6.3 kPa = 47 mmHg. It also CO 2
increases the concentration of CO 2 dissolved in
Arterial blood:
plasma. However, the major portion of the CO 2 Plasma* 0.7 13.2 0.1 14.0
diffuses into red blood cells, thereby increas- Erythrocytes** 0.5 6.5 1.1 8.1
ing their content of dissolved CO 2. CO 2 (+ H 2O)
within the cells is converted to HCO 3 (! A5, 2) Blood 1.2 19.7 1.2 22.1
–
– Mixed venous blood:
and hemoglobin carbamate (! A3). The HCO 3
concentration in erythrocytes therefore be- Plasma* 0.8 14.3 ca. 0.1 15.2
comes higher than in plasma. As a result, about Erythrocytes** 0.6 7.2 1.4 9.2
– Blood 1.4 21.5 1.5 24.4
three-quarters of the HCO 3 ions exit the
–
–
erythrocytes by way of an HCO 3 /Cl anti- Arteriovenous CO 2 difference in blood
porter. This anion exchange is also called Ham- 0.2 1.8 0.3 2.3
burger shift (! A4). Percentage of total arteriovenous difference
H ions are liberated when CO 2 in red cells 9% 78% 13% 100%
+
circulating in the periphery is converted to * Approx 0.55 L plasma/L blood; ** ca. 0.45 L
–
HCO 3 and hemoglobin (Hb) carbamate. erythrocytes/L blood
Bicarbonate formation:
–
CO 2 + H 2O HCO 3 + H , + [5.4]
Hemoglobin carbamate formation:
–
+
Hb–NH 2 + CO 2 Hb–NH–COO + H . [5.5]
+
Hemoglobin (Hb) is a key buffer for H ions in
the red cells (! A6; see also p. 140, “Non-bicar-
124 bonate buffers”). Since the removal of H ions
+
Despopoulos, Color Atlas of Physiology © 2003 Thieme
All rights reserved. Usage subject to terms and conditions of license.