Page 154 - Color_Atlas_of_Physiology_5th_Ed._-_A._Despopoulos_2003
P. 154
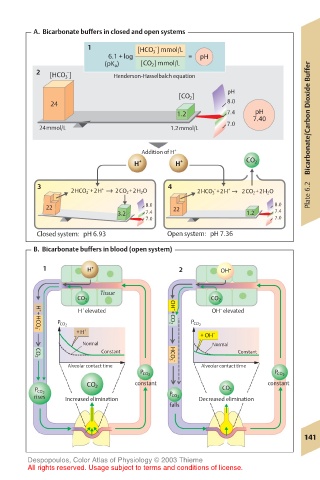
A. Bicarbonate buffers in closed and open systems
1 [HCO 3 ] mmol/L
–
6.1 + log = pH
(pK a ) [CO 2 ] mmol/L
2 [HCO 3 ] Henderson-Hasselbalch equation
–
pH
[CO 2 ]
24 8.0
1.2 7.4 pH
7.40 Bicarbonate/Carbon Dioxide Buffer
7.0
24mmol/L 1.2mmol/L
Addition of H +
H + H + CO 2
3 – + 4
+
2HCO 3 +2H → 2CO 2 +2H 2 O 2HCO 3 +2H → 2CO 2 +2H 2 O Plate 6.2
–
22 8.0 22 8.0
3.2 7.4 1.2 7.4
7.0 7.0
Closed system: pH 6.93 Open system: pH 7.36
B. Bicarbonate buffers in blood (open system)
1 H + 2 OH –
Tissue
CO 2 CO 2
–
+
H elevated OH – +CO 2 OH elevated
P CO 2 P CO 2
+H + + OH –
H + +HCO 3 –
Normal Normal
Constant HCO 3 – Constant
CO 2
Alveolar contact time Alveolar contact time
P CO 2 P CO 2
constant constant
CO 2
CO 2
P CO 2
rises Increased elimination P CO 2 Decreased elimination
falls
141
Despopoulos, Color Atlas of Physiology © 2003 Thieme
All rights reserved. Usage subject to terms and conditions of license.