Page 584 - Clinical Application of Mechanical Ventilation
P. 584
550 Chapter 17
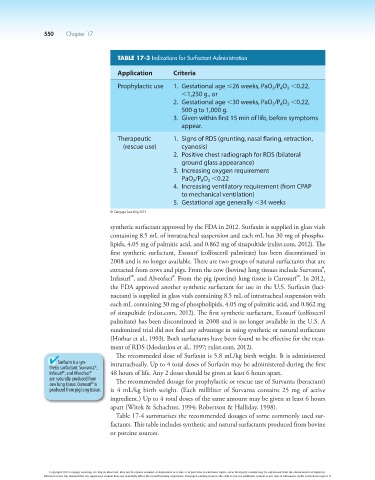
TABLE 17-3 Indications for Surfactant Administration
Application Criteria
Prophylactic use 1. Gestational age #26 weeks, PaO /P O ,0.22,
A
2
2
,1,250 g., or
2. Gestational age ,30 weeks, PaO /P O ,0.22,
2
2
A
500 g to 1,000 g.
3. Given within first 15 min of life, before symptoms
appear.
Therapeutic 1. Signs of RDS (grunting, nasal flaring, retraction,
(rescue use) cyanosis)
2. Positive chest radiograph for RDS (bilateral
ground glass appearance)
3. Increasing oxygen requirement
PaO /P O ,0.22
2
2
A
4. Increasing ventilatory requirement (from CPAP
to mechanical ventilation)
5. Gestational age generally ,34 weeks
© Cengage Learning 2014
synthetic surfactant approved by the FDA in 2012. Surfaxin is supplied in glass vials
containing 8.5 mL of intratracheal suspension and each mL has 30 mg of phospho-
lipids, 4.05 mg of palmitic acid, and 0.862 mg of sinapultide (rxlist.com, 2012). The
first synthetic surfactant, Exosurf (colfosceril palmitate) has been discontinued in
2008 and is no longer available. There are two groups of natural surfactants that are
extracted from cows and pigs. From the cow (bovine) lung tissues include Survanta®,
Infasurf ®, and Alveofact®. From the pig (porcine) lung tissue is Curosurf®. In 2012,
the FDA approved another synthetic surfactant for use in the U.S. Surfaxin (luci-
nactant) is supplied in glass vials containing 8.5 mL of intratracheal suspension with
each mL containing 30 mg of phospholipids, 4.05 mg of palmitic acid, and 0.862 mg
of sinapultide (rxlist.com, 2012). The first synthetic surfactant, Exosurf (colfosceril
palmitate) has been discontinued in 2008 and is no longer available in the U.S. A
randomized trial did not find any advantage in using synthetic or natural surfactant
(Horbar et al., 1993). Both surfactants have been found to be effective for the treat-
ment of RDS (Modanlou et al., 1997; rxlist.com, 2012).
The recommeded dose of Surfaxin is 5.8 mL/kg birth weight. It is administered
Surfaxin is a syn- intratracheally. Up to 4 total doses of Surfaxin may be administered during the first
thetic surfactant. Survanta®,
Infasurf®, and Alveofact® 48 hours of life. Any 2 doses should be given at least 6 hours apart.
are naturally produced from The recommended dosage for prophylactic or rescue use of Survanta (beractant)
cow lung tissue. Curosurf® is
produced from pig lung tissue. is 4 mL/kg birth weight. (Each milliliter of Survanta contains 25 mg of active
ingredient.) Up to 4 total doses of the same amount may be given at least 6 hours
apart (Witek & Schachter, 1994; Robertson & Halliday, 1998).
Table 17-4 summarizes the recommended dosages of some commonly used sur-
factants. This table includes synthetic and natural surfactants produced from bovine
or porcine sources.
Copyright 2013 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s).
Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it.