Page 1073 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1073
956 Part VII Hematologic Malignancies
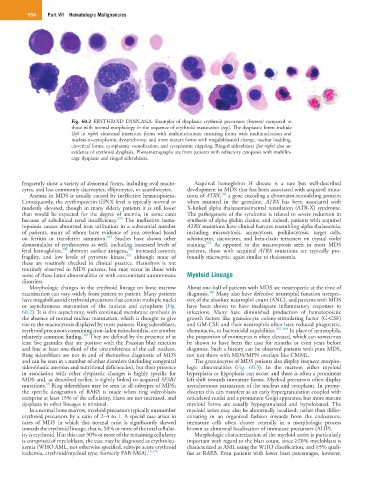
Fig. 60.2 ERYTHROID DYSPLASIA. Examples of dysplastic erythroid precursors (bottom) compared to
those with normal morphology in the sequence of erythroid maturation (top). The dysplastic forms include
(left to right) abnormal immature forms with multinucleation; maturing forms with multinucleation and
nuclear-to-cytoplasmic dyssynchrony; and more mature forms with megaloblastoid change, nuclear budding,
cloverleaf forms, cytoplasmic vacuolization, and cytoplasmic stippling. Ringed sideroblasts (far right) also are
evidence of erythroid dysplasia. Photomicrographs are from patients with refractory cytopenia with multilin-
eage dysplasia and ringed sideroblasts.
frequently show a variety of abnormal forms, including oval macro- Acquired hemoglobin H disease is a rare but well-described
cytes, and less commonly dacrocytes, elliptocytes, or acanthocytes. development in MDS that has been associated with acquired muta-
304
Anemia in MDS is usually caused by ineffective hematopoiesis. tions of ATRX, a gene encoding a chromatin-remodeling protein;
Consequently, the erythropoietin (EPO) level is typically normal or when mutated in the germline, ATRX has been associated with
modestly elevated, though in many elderly patients it is still lower X-linked alpha thalassemia/mental retardation (ATR-X) syndrome.
than would be expected for the degree of anemia, in some cases The pathogenesis of the syndrome is related to severe reduction in
298
because of subclinical renal insufficiency. The ineffective hema- synthesis of alpha globin chains, and indeed, patients with acquired
topoiesis causes abnormal iron utilization in a substantial number ATRX mutations have clinical features resembling alpha thalassemia,
of patients, many of whom have evidence of iron overload based including microcytosis, anisocytosis, poikilocytosis, target cells,
299
on ferritin or transferrin saturation. Studies have shown other schistocytes, dacrocytes, and beta-chain tetramers on crystal violet
305
abnormalities of erythrocytes as well, including increased levels of staining. As opposed to the macrocytosis seen in most MDS
301
300
fetal hemoglobin, aberrant surface antigens, increased osmotic patients, those with acquired ATRX mutations are typically pro-
302
fragility, and low levels of pyruvate kinase, although none of foundly microcytic, again similar to thalassemia.
these are routinely checked in clinical practice. Hemolysis is not
routinely observed in MDS patients, but may occur in those with
some of these latter abnormalities or with concomitant autoimmune Myeloid Lineage
disorders.
Morphologic changes in the erythroid lineage on bone marrow About one-half of patients with MDS are neutropenic at the time of
306
examination can vary widely from patient to patient. Many patients diagnosis. Many also have defective neutrophil function irrespec-
have megaloblastoid erythroid precursors that contain multiple nuclei tive of the absolute neutrophil count (ANC), and patients with MDS
or asynchronous maturation of the nucleus and cytoplasm (Fig. have been shown to have inadequate inflammatory responses to
60.2). It is this asynchrony, with continued membrane synthesis in infections. Many have diminished production of hematopoietic
the absence of normal nuclear maturation, which is thought to give growth factors like granulocyte colony-stimulating factor (G-CSF)
rise to the macrocytosis displayed by most patients. Ring sideroblasts, and GM-CSF, and their neutrophils often have reduced phagocytic,
erythroid precursors containing iron-laden mitochondria, are another chemotactic, or bactericidal capabilities. 307–309 In place of neutrophils,
303
relatively common finding. They are defined by the presence of at the proportion of monocytes is often elevated, which can sometimes
least five granules that are positive with the Prussian blue reaction be shown to have been the case for months or even years before
and line at least one-third of the circumference of the cell nucleus. diagnosis. Such a history can be observed patients with pure MDS,
Ring sideroblasts are not in and of themselves diagnostic of MDS not just those with MDS/MPN overlaps like CMML.
and can be seen in a number of other disorders (including congenital The granulocytes of MDS patients also display frequent morpho-
sideroblastic anemias and nutritional deficiencies), but their presence logic abnormalities (Fig. 60.3). In the marrow, either myeloid
in association with other dysplastic changes is highly specific for hyperplasia or hypoplasia can occur, and there is often a prominent
MDS and, as described earlier, is tightly linked to acquired SF3B1 left shift towards immature forms. Myeloid precursors often display
88
mutations. Ring sideroblasts may be seen in all subtypes of MDS; asynchronous maturation of the nucleus and cytoplasm. In promy-
the specific designation of RARS is made when ring sideroblasts elocytes this can manifest as an early hypergranulation coupled with
comprise at least 15% of the cellularity, blasts are not increased, and reticulated nuclei and a prominent Golgi apparatus, but more mature
dysplasia in other lineages is minimal. myeloid forms are usually hypogranulated and hypolobated. The
In a normal bone marrow, myeloid precursors typically outnumber myeloid series may also be abnormally localized: rather than differ-
erythroid precursors by a ratio of 2–4 to 1. A special case arises in entiating in an organized fashion inwards from the endosteum,
cases of MDS in which this normal ratio is significantly skewed immature cells often cluster centrally in a morphologic process
towards the erythroid lineage, that is, 50% or more of the total cellular- known as abnormal localization of immature precursors (ALIP).
ity is erythroid. If in this case 30% or more of the remaining cellularity Morphologic characterization of the myeloid series is particularly
is comprised of myeloblasts, the case may be diagnosed as erythroleu- important with regard to the blast count, since ≥20% myeloblasts is
kemia (WHO AML, not otherwise specified, subtype acute erythroid characterized as AML using the WHO classification, and ≥5% quali-
leukemia, erythroid/myeloid type; formerly FAB-M6A). 15,170 fies as RAEB. Even patients with lower blast percentages, however,