Page 1105 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1105
Chapter 61 Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome in Adults 975
100 100
1970-79 1995-99 75 1970-79 1995-99
Survival still alive (%) 50 53 43 1990-95 29 Survival still alive (%) 50 1990-95
2000-04
2000-04
1980-84
1980-84
75
1985-89
2005-09
2005-09
1985-89
35
25
25
5
0 23 15 0 23 10 7 4 2 2 1
0 5 10 15 20 25 0 5 10 15 20 25
A Time from entry (years) B Time from entry (years)
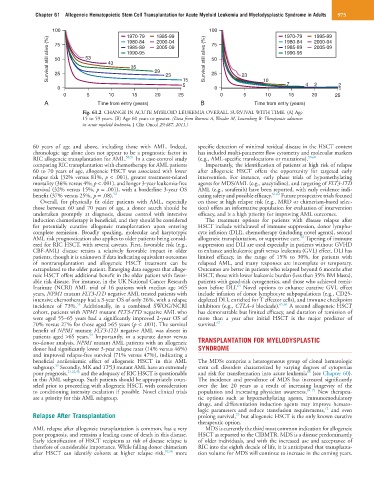
Fig. 61.2 CHANGE IN ACUTE MYELOID LEUKEMIA OVERALL SURVIVAL WITH TIME. (A) Age
15 to 59 years. (B) Age 60 years or greater. (Data from Burnett A, Wetzler M, Lowenberg B: Therapeutic advances
in acute myeloid leukemia, J Clin Oncol 29:487, 2011.)
60 years of age and above, including those with AML. Indeed, specific detection of minimal residual disease in the HSCT context
chronologic age alone does not appear to be a prognostic factor in has included multi-parameter flow cytometry and molecular markers
RIC allogeneic transplantation for AML. 50,51 In a case-control study (e.g., AML-specific translocations or mutations). 59,60
comparing RIC transplantation with chemotherapy for AML patients Importantly, the identification of patients at high risk of relapse
60 to 70 years of age, allogeneic HSCT was associated with lower after allogeneic HSCT offers the opportunity for targeted early
relapse risk (32% versus 81%, p < .001), greater treatment-related intervention. For instance, early phase trials of hypomethylating
mortality (36% versus 4%; p < .001), and longer 3-year leukemia-free agents for MDS/AML (e.g., azacytidine), and targeting of FLT3-ITD
survival (32% versus 15%, p = .001), with a borderline 3-year OS AML (e.g., sorafenib) have been reported, with early evidence indi-
benefit (37% versus 25%, p = .08). 52 cating safety and possible efficacy. 61,62 Future prospective trials focused
Overall, for physically fit older patients with AML, especially on those at high relapse risk (e.g., MRD or chimerism-based selec-
those between 60 and 70 years of age, a donor search should be tion) offers an informative population for evaluation of intervention
undertaken promptly at diagnosis, disease control with intensive efficacy, and is a high priority for improving AML outcomes.
induction chemotherapy is beneficial, and they should be considered The treatment options for patients with disease relapse after
for potentially curative allogeneic transplantation upon entering HSCT include withdrawal of immune suppression, donor lympho-
complete remission. Broadly speaking, molecular and karytotypic cyte infusion (DLI), chemotherapy (including novel agents), second
63
AML risk prognostication also applies to older patients being consid- allogeneic transplantation, or supportive care. Tapering of immune
ered for RIC HSCT, with several caveats. First, favorable risk (e.g., suppression and DLI are used especially in patients without GVHD
CBF-AML) disease retains a relatively favorable impact in older to enhance antileukemic graft versus leukemia (GVL) effect. DLI has
patients, though it is unknown if data indicating equivalent outcomes limited efficacy, in the range of 15% to 30%, for patients with
of nontransplantation and allogeneic HSCT treatment can be relapsed AML, and many responses are incomplete or temporary.
extrapolated to the older patient. Emerging data suggests that alloge- Outcomes are better in patients who relapsed beyond 6 months after
neic HSCT offers additional benefit in the older patient with favor- HSCT, those with lower leukemic burden (less than 35% BM blasts),
able risk disease. For instance, in the UK National Cancer Research patients with good-risk cytogenetics, and those who achieved remis-
64
Institute (NCRI) AML trial of 16 patients with median age >65 sion before DLI. Novel options to enhance curative GVL effect
years, NPM1 mutant FLT3-ITD negative AML treated patients with include infusion of donor lymphocyte subpopulations (e.g., CD25-
intensive chemotherapy had a 3-year OS of only 26%, with a relapse depleted DLI, enriched for T effector cells), and immune checkpoint
53
incidence of 73%. Additionally, in a combined SWOG/NCRI inhibitors (e.g., CTLA-4 blockade). 65,66 A second allogeneic HSCT
cohort, patients with NPM1 mutant FLT3-ITD negative AML who has demonstrable but limited efficacy, and duration of remission of
were aged 55−65 years had a significantly improved 2-year OS of more than a year after initial HSCT is the major predictor of
70% versus 27% for those aged >65 years (p < .001). The survival survival. 67
benefit of NPM1 mutant FLT3-ITD negative AML was absent in
54
patients aged >65 years. Importantly, in a separate donor versus
no-donor analysis, NPM1 mutant AML patients with an allogeneic TRANSPLANTATION FOR MYELODYSPLASTIC
donor had significantly lower 3-year relapse rates (14% versus 46%) SYNDROME
and improved relapse-free survival (71% versus 47%), indicating a
beneficial antileukemic effect of allogeneic HSCT in this AML The MDSs comprise a heterogeneous group of clonal hematologic
55
subgroup. Secondly, MK and TP53 mutant AML have an extremely stem cell disorders characterized by varying degrees of cytopenias
68
poor prognosis, 21,22,56 and the adequacy of RIC HSCT is questionable and risk for transformation into acute leukemia (see Chapter 60).
in this AML subgroup. Such patients should be appropriately coun- The incidence and prevalence of MDS has increased significantly
seled prior to proceeding with allogeneic HSCT, with consideration over the last 20 years as a result of increasing longevity of the
to conditioning intensity escalation if possible. Novel clinical trials population and increasing physician awareness. 69–71 New therapeu-
are a priority for this AML subgroup. tic options such as hypomethylating agents, immunomodulatory
drugs, and differentiation induction agents may improve hemato-
72
logic parameters and reduce transfusion requirements, and even
Relapse After Transplantation prolong survival, but allogeneic HSCT is the only known curative
73
therapeutic option.
AML relapse after allogeneic transplantation is common, has a very MDS is currently the third most common indication for allogeneic
poor prognosis, and remains a leading cause of death in this disease. HSCT as reported to the CIBMTR. MDS is a disease predominantly
Early identification of HSCT recipients at risk of disease relapse is of older individuals, and with the increased use and acceptance of
therefore of considerable importance. While falling donor chimerism RIC into the eighth decade of life, it is anticipated that transplanta-
after HSCT can identify cohorts at higher relapse risk, 57,58 more tion volume for MDS will continue to increase in the coming years.