Page 1308 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1308
1154 Part VII Hematologic Malignancies
eosinophil eicosanoide-forming enzymes, including 5-lipoxygenase, Monocytes
leukotriene C4 synthase, and cyclooxygenase (see Table 71.1). 100
The immunophenotype of human eosinophils is well established
and may assist in eosinophil detection and enumeration by flow 80
cytometry. In common with all leukocytes, eosinophils express leu- 60
kosialin (CD43), the homing cell adhesion molecule HCAM (CD44),
5
and the pan-leukocyte tyrosine phosphatase C (CD45). In addition, Relative cell number (%) 40
eosinophils express several myeloid differentiation antigens, including
LFA-1 (CD11a/CD18) and Siglec-3 (CD33). These cell surface 20
antigens are also expressed on other myeloid cells, including mono-
cytes and basophils. However, a few cell surface structures are largely 0
restricted to eosinophils and thus serve as cell-specific markers 10 0 10 1 10 2 10 3 10 4
through which eosinophils can be detected and isolated. One of these Siglec-8
markers is Siglec-8, an inhibitory receptor that, when crosslinked,
6
mediates eosinophil apoptosis. Apart from eosinophils, basophils Basophils
also display Siglec-8 (Fig. 71.1). Eosinophils also express several 100
cytokine receptors, such as the IL-5 receptor, granulocyte-macrophage
colony-stimulating factor (GM-CSF) receptor, and IL-3 receptor. 80
Moreover, eosinophils exhibit various chemokine receptors, including
eotaxin receptors and CXC-chemokine receptor 4 (CXCR4), a recep- 60
tor for stromal cell-derived factor (SDF)-1 (Table 71.5). Finally, Relative cell number (%)
eosinophils express diverse complement receptors (CRs), such as CR3 40
(CD11b/CD18) and C5aR (CD88), adhesion receptors, and immu- 20
noglobulin (Fc) receptors. Several of these cell surface markers have
been considered as potential targets of therapy. However, no aber- 0
rantly expressed or disease-specific markers for neoplastic eosinophils 10 0 10 1 10 2 10 3 10 4
or reactive eosinophils have been identified yet. Several studies have
shown that eosinophils in HES patients may express increased Siglec-8
amounts of CD11b, CD16, CD25, or/and HLA-DR. However, Eosinophils
overexpression of these antigens is not disease-specific. 100
Apart from cell surface antigens, eosinophils also express more or
less specific marker antigens in their cytoplasm by which these cells 80
can be detected in various tissues by immunohistochemistry (IHC).
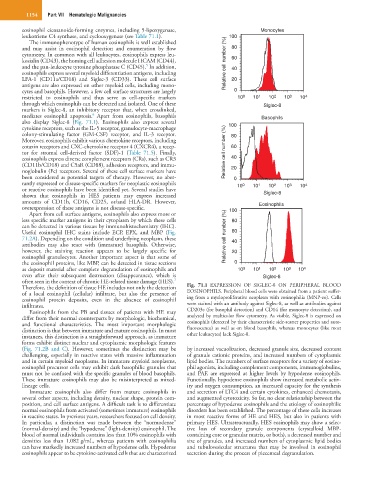
Useful eosinophil IHC stains include ECP, EPX, and MBP (Fig. 60
71.2A). Depending on the condition and underlying neoplasm, these Relative cell number (%) 40
antibodies may also react with (immature) basophils. Otherwise,
however, the staining reaction appears to be largely specific for 20
eosinophil granulocytes. Another important aspect is that some of
the eosinophil proteins, like MBP, can be detected in tissue sections 0
as deposit material after complete degranulation of eosinophils and 10 0 10 1 10 2 10 3 10 4
even after their subsequent destruction (disappearance), which is Siglec-8
7
often seen in the context of chronic HE-related tissue damage (HES).
Therefore, the definition of tissue HE includes not only the detection Fig. 71.1 EXPRESSION OF SIGLEC-8 ON PERIPHERAL BLOOD
of a local eosinophil (cellular) infiltrate, but also the presence of EOSINOPHILS. Peripheral blood cells were obtained from a patient suffer-
eosinophil protein deposits, even in the absence of eosinophil ing from a myeloproliferative neoplasm with eosinophilia (MNP-eo). Cells
infiltrates. were stained with an antibody against Siglec-8, as well as antibodies against
Eosinophils from the PB and tissues of patients with HE may CD203c (for basophil detection) and CD14 (for monocyte detection), and
differ from their normal counterparts by morphologic, biochemical, analyzed by multicolor flow cytometry. As visible, Siglec-8 is expressed on
and functional characteristics. The most important morphologic eosinophils (detected by their characteristic side-scatter properties and auto-
distinction is that between immature and mature eosinophils. In most fluorescence) as well as on blood basophils, whereas monocytes (like most
instances, this distinction is a straightforward approach, as immature other leukocytes) lack Siglec-8.
forms exhibit distinct nuclear and cytoplasmic morphologic features
(Fig. 71.2B and C). However, sometimes the distinction may be by increased vacuolization, decreased granule size, decreased content
challenging, especially in reactive states with massive inflammation of granule cationic proteins, and increased numbers of cytoplasmic
and in certain myeloid neoplasms. In immature myeloid neoplasms, lipid bodies. The numbers of surface receptors for a variety of eosino-
eosinophil precursor cells may exhibit dark basophilic granules that phil agonists, including complement components, immunoglobulins,
must not be confused with the specific granules of blood basophils. and PAF, are expressed at higher levels by hypodense eosinophils.
These immature eosinophils may also be misinterpreted as mixed- Functionally, hypodense eosinophils show increased metabolic activ-
lineage cells. ity and oxygen consumption, an increased capacity for the synthesis
Immature eosinophils also differ from mature eosinophils in and secretion of LTC4 and certain cytokines, enhanced chemotaxis,
several other aspects, including density, nuclear shape, protein com- and augmented cytotoxicity. So far, no clear relationship between the
position, and cell surface antigens. A difficult task is to differentiate percentage of hypodense eosinophils and the etiology of eosinophilic
normal eosinophils from activated (sometimes immature) eosinophils disorders has been established. The percentage of these cells increases
in reactive states. In previous years, researchers focused on cell density. in most reactive forms of HE and HES, but also in patients with
In particular, a distinction was made between the “normodense” primary HES. Ultrastructurally, HES eosinophils may show a selec-
(normal-density) and the “hypodense” (light-density) eosinophil. The tive loss of secondary granule components (crystalloid MBP-
blood of normal individuals contains less than 10% eosinophils with containing core or granular matrix, or both), a decreased number and
densities less than 1.082 g/mL, whereas patients with eosinophilia size of granules, and increased numbers of cytoplasmic lipid bodies
can have markedly increased numbers of hypodense cells. Hypodense and tubulovesicular structures that may be involved in eosinophil
eosinophils appear to be cytokine-activated cells that are characterized secretion during the process of piecemeal degranulation.