Page 1309 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1309
Chapter 71 Eosinophilia, Eosinophil-Associated Diseases, Eosinophilic Leukemias, and the Hypereosinophilic Syndromes 1155
TABLE Receptors and Ligands Regulating Growth and
71.5 Function of Eosinophils
Receptor (R) on
Eosinophils Ligand Effects on Eosinophils
IL-2RA/CD25 a IL-2 Activation? Migration? a
a
IL-3R/CD123 + IL-3 Differentiation, survival,
CD131 adhesion, migration,
activation, priming
IL-4R/CD124 IL-4 Priming for effects of
chemotaxins
IL-5R/CD125 + IL-5 Differentiation, survival,
CD131 adhesion, migration,
activation, priming A
GM-CSFR/CD116 GM-CSF Differentiation, survival,
+ CD131 adhesion, migration,
activation, priming
IL-10R IL-10 Inhibitory (activation,
survival)
IL-12R IL-12 Inhibitory (activation)
IL-13R IL-13 Unknown
CD4 IL-16 (LCF) Activation, priming
IL-25R IL-25 Survival, activation
IL-27R IL-27 Survival, activation
IL-33R/ST2 IL-33 Activation, survival
VEGFR-1/FLT-1 VEGF Chemotaxis, activation
Tie-2/TEK Angiopoietin-1 Chemotaxis, activation? B
PDGFRA/B PDGF Activation and growth?
FGFR FGF Activation?
TGFßβ1R TGFβ1 Inhibitory (differentiation)
TGFβ2R TGFβ2 Inhibitory (differentiation)
IFN-α-R IFN-α Inhibitory (growth)
IFN-γ-R IFN-γ Inhibitory (growth,
migration)
CCR3 (CD193) RANTES (CCL5) Chemotaxis, activation
MCP-3 (CCL7) Chemotaxis, activation
MCP-4 (CCL13) Chemotaxis, activation
Eotaxin-1 (CCL11) Chemotaxis, activation
Eotaxin-2 (CCL24) Chemotaxis, activation
Eotaxin-3 (CCL26) Chemotaxis, activation
C
CXCR4 (CD184) SDF-1 (CXCL12) Chemotaxis
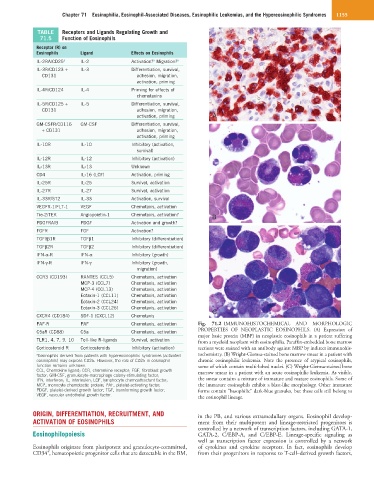
PAF-R PAF Chemotaxis, activation Fig. 71.2 IMMUNOHISTOCHEMICAL AND MORPHOLOGIC
PROPERTIES OF NEOPLASTIC EOSINOPHILS. (A) Expression of
C5aR (CD88) C5a Chemotaxis, activation
major basic protein (MBP) in neoplastic eosinophils in a patient suffering
TLR1, 4, 7, 9, 10 Toll-like R-ligands Survival, activation from a myeloid neoplasm with eosinophilia. Paraffin-embedded bone marrow
Corticosteroid R Corticosteroids Inhibitory (activation) sections were stained with an antibody against MBP by indirect immunohis-
a Eosinophils derived from patients with hypereosinophilic syndromes (activated tochemistry. (B) Wright-Giemsa-stained bone marrow smear in a patient with
eosinophils) may express CD25. However, the role of CD25 in eosinophil chronic eosinophilic leukemia. Note the presence of atypical eosinophils,
function remains unknown. some of which contain multi-lobed nuclei. (C) Wright-Giemsa-stained bone
CCL, Chemokine ligand; CCR, chemokine receptor; FGF, fibroblast growth marrow smear in a patient with an acute eosinophilic leukemia. As visible,
factor; GM-CSF, granulocyte-macrophage colony-stimulating factor;
IFN, interferon; IL, interleukin; LCF, lymphocyte chemoattractant factor; the smear contains a mixture of immature and mature eosinophils. Some of
MCP, monocyte chemotactic protein; PAF, platelet-activating factor; the immature eosinophils exhibit a blast-like morphology. Other immature
PDGF, platelet-derived growth factor; TGF, transforming growth factor; forms contain “basophilic” dark-blue granules, but these cells still belong to
VEGF, vascular endothelial growth factor.
the eosinophil lineage.
ORIGIN, DIFFERENTIATION, RECRUITMENT, AND in the PB, and various extramedullary organs. Eosinophil develop-
ACTIVATION OF EOSINOPHILS ment from their multipotent and lineage-restricted progenitors is
controlled by a network of transcription factors, including GATA-1,
Eosinophilopoiesis GATA-2, C/EBP-A, and C/EBP-E. Lineage-specific signaling as
well as transcription factor expression is controlled by a network
Eosinophils originate from pluripotent and granulocyte-committed, of cytokines and cytokine receptors. In fact, eosinophils develop
+
CD34 , hematopoietic progenitor cells that are detectable in the BM, from their progenitors in response to T-cell–derived growth factors,