Page 1469 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1469
1306 Part VII Hematologic Malignancies
versus ASCT resulted in a greater mortality at 100 days (19% vs. 0%, LR median not reached
p < .01), numerically lower but statistically similar relapse rate (21% 1.0 IR median = 51
45
vs. 56%, p = .11) and similar OS (49% vs. 47%, p = .51) at 5 years.
Similarly, in a Center for International Blood and Marrow Transplant 0.9 HR median = 29
Research (CIBMTR) study of chemosensitive patients with MCL 0.8
(n = 519), reduced intensity conditioning (RIC) alloSCT, compared 0.7
with ASCT, resulted in lower relapse/progression, which was offset
by a higher nonrelapse mortality (NRM); hence, the 5-year OS was 0.6
similar between the two groups. ASCT in first complete remission Probability of overall survival 0.5
was associated with the highest OS and PFS confirming the results 0.4
60
of prior studies of upfront HDT/ASCT. The comparable OS with
alloSCT, compared with ASCT, in these studies also demonstrates 0.3
the efficacy of disease control with alloSCT in patients with multiple 0.2
relapses. In clinical practice, therefore, alloSCT may be utilized in 0.1
heavily pretreated patients. 0.0
RIC has been explored with a hope to reduce TRM and expand
alloSCT to older patients or patients with other comorbidities. In a 0 12 24 36 48 60 72 84 96
study, 70 patients with relapsed and refractory MCL including prior Months since registration
ASCT failures (34%) underwent RIC alloSCT, mainly with
alemtuzumab-containing regimens. NRM, cumulative risk of relapse, Numbers of patients at risk
PFS, and OS were 21%, 65%, 14%, and 37%, respectively, at 5 years. LR 180 153 131 99 69 39 15 4
116
83
IR
145
57
19
37
9
5
Among patients who relapsed, donor lymphocyte infusion (DLI) or HR 84 58 29 19 8 5 1 0
61
a second RIC alloSCT resulted in a complete remission rate of 73%.
In another study of 33 relapsed and patients with refractory MCL, Fig. 81.5 PROGNOSTIC GROUPS STRATIFIED ACCORDING TO
nonmyeloablative conditioning with fludarabine and 2 Gy total body THE MANTLE CELL INTERNATIONAL PROGNOSTIC INDEX.
irradiation was associated with an overall response rate of 85%, 2-year HR, High risk; IR, intermediate risk; LR, low risk.
NRM, disease-free survival, and OS of 24%, 60%, and 65% respec-
62
tively. Hence, RIC and nonmyeloablative alloSCT along with DLI expression profiling, genome-wide miRNA profiling as well as a
for relapse may be reasonable options in patients who are not candi- limited-gene model (based on five genes RAN, MYC, TNFRSF10B,
dates for myeloablative alloSCT. POLE2, and SLC29A2) may also provide meaningful prognostic
information; however, the lack of commercial testing and standard-
ization, as well as high cost limit their use in clinical practice.
PROGNOSIS
As discussed previously, complete remission is seen in up to two-third FUTURE DIRECTIONS
of patients with MCL, particularly with upfront intensive therapy;
however, almost all patients will relapse and develop resistance to Since the recognition of MCL as a separate entity there has been
chemotherapy over time. Although the prognosis for newly diagnosed significant advancement in the understanding of tumor biology,
patients has improved over the last decades, the OS compares unfa- diagnostic, prognostic, and treatment approaches. This has resulted
vorably with the more frequent NHLs such as follicular lymphoma in a significant improvement in OS of patients with MCL at a popu-
and diffuse large B-cell lymphoma. A small subset of patients may lation level, with approximately half of the patients in the United
65
follow a more indolent clinical course in the beginning, and these States surviving beyond 5 years in recent years. A few studies have
patients may have a clinical history that resembles a chronic lympho- demonstrated poor prognosis of blastoid-variant MCL. Intensified
cytic leukemia. therapy including upfront ASCT may overcome this poor prognosis,
Prior studies have identified several adverse prognostic factors, which needs further exploration in prospective studies. The roles of
which include eastern cooperative oncology group (ECOG) perfor- radiotherapy and CNS prophylaxis are currently not well established
mance status (PS) ≥2, age >65 years, elevated LDH, elevated serum and should be investigated. Integration of clinical parameters and
beta 2 -microglobulin, hemoglobin level <12 g/dL, advanced stage, gene expression profiling has a potential to improve prognostication.
involvement of peripheral blood or extranodal sites ≥2, high cell Such prognostication may identify an indolent subgroup that can be
proliferation rate (Ki-67, mitotic index or proliferation signature), observed, as well as an aggressive subgroup that may be better served
blastoid variant histology, deletion or mutation of TP53, and second- with upfront intensive therapy. A development of prognosis-directed
ary chromosomal aberrations (the presence of gain 3q, 12q and therapy is an ideal goal. Molecular markers such as t(11;14), clonal
63
13q and losses of 6q, 9p, 11q, and 17p). More recent studies have immunoglobulin heavy chain gene rearrangements, and SOX11 gene
demonstrated that the MCL international prognostic index (MIPI), expression may be used to identify minimal residual disease. Although
based on age, ECOG PS, LDH, and white blood cell count, is one the correlation is not consistent, molecular remission has been cor-
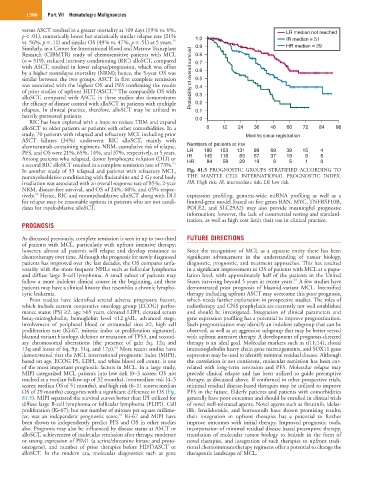
of the most important prognostic factors in MCL. In a large study, related with long-term remission and PFS. Molecular relapse may
MIPI categorized MCL patients into low risk (0–3 scores; OS not precede clinical relapse and has been utilized to guide preemptive
reached at a median follow-up of 32 months), intermediate risk (4–5 therapy, as discussed above. If confirmed in other prospective trials,
scores; median OS of 51 months), and high risk (6–11 scores; median minimal residual disease-based therapies may be utilized to improve
OS of 29 months) categories with a significant difference in OS (Fig. PFS in the future. Elderly patients and patients with comorbidities
81.5). MIPI separated the survival curves better than IPI utilized for generally have poor outcomes and should be enrolled in clinical trials
diffuse large B-cell lymphoma or follicular lymphoma (FLIPI). Cell of novel well-tolerated agents. Novel agents such as ibrutinib, idelas-
proliferation (Ki-67), but not number of mitoses per square millime- ilib, lenalidomide, and bortezomib have shown promising results;
64
ter, was an independent prognostic score. Ki-67 and MIPI have their integration in upfront therapies has a potential to further
been shown to independently predict PFS and OS in other studies improve outcomes with initial therapy. Improved prognostic tools,
also. Prognosis may also be influenced by disease status at ASCT or incorporation of minimal residual disease based preemptive therapy,
alloSCT, achievement of molecular remission after therapy, moderate translation of molecular tumor biology to bedside in the form of
or strong expression of PIM1 (a serine/threonine kinase and proto- novel therapies, and integration of such therapies to upfront tradi-
oncogene), and number of prior therapies before HDT/ASCT or tional chemoimmunotherapy regimens offer a potential to change the
alloSCT. In the modern era, molecular diagnostics such as gene therapeutic landscape of MCL.