Page 1468 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1468
Chapter 81 Mantle Cell Lymphoma 1305
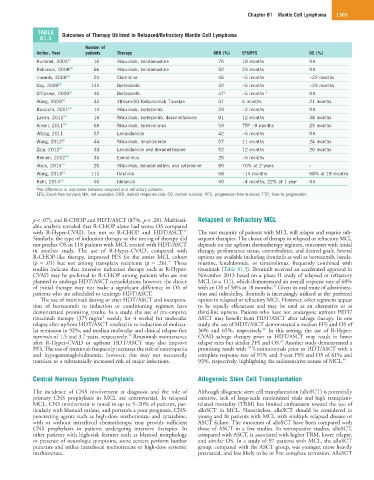
TABLE Outcomes of Therapy Utilized in Relapsed/Refractory Mantle Cell Lymphoma
81.3
Number of
Author, Year patients Therapy ORR (%) EFS/PFS OS (%)
Rummel, 2005 47 16 Rituximab, bendamustine 75 18 months NA
Robinson, 2008 48 66 Rituximab, bendamustine 92 23 months NA
Inwards, 2008 49 24 Cladribine 46 ~5 months ~22 months
Goy, 2009 50 141 Bortezomib 32 ~6 months ~23 months
O’Connor, 2009 51 40 Bortezomib 47 a ~5 months a NA
Wang, 2009 52 32 Yttrium-90-Ibritumomab Tiuxetan 31 6 months 21 months
Baiocchi, 2011 53 14 Rituximab, bortezomib 29 ~2 months NA
Lamm, 2011 54 16 Rituximab, bortezomib, dexamethasone 81 12 months 38 months
Ansell, 2011 55 69 Rituximab, temsirolimus 59 TTP ~9 months 29 months
Witzig, 2011 57 Lenalidomide 42 ~6 months NA
Wang, 2012 56 44 Rituximab, lenalidomide 57 11 months 24 months
Zaja, 2012 57 33 Lenalidomide and dexamethasone 52 12 months 20 months
Renner, 2012 58 35 Everolimus 20 ~5 months
Visco, 2013 15 20 Rituximab, bendamustine, and cytarabine 80 70% at 2 years -
Wang, 2013 43 111 Ibrutinib 68 ~14 months 58% at 18 months
Kahl, 2014 59 40 Idelalisib 40 ~4 months; 22% at 1 year NA
a No difference in outcomes between relapsed and refractory patients.
EFS, Event-free survival; NA, not available; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression
p < .07), and R-CHOP and HDT/ASCT (87%, p < .20). Multivari- Relapsed or Refractory MCL
able analysis revealed that R-CHOP alone had worse OS compared
40
with R-Hyper-CVAD, but not to R-CHOP and HDT/ASCT. The vast majority of patients with MCL will relapse and require sub-
Similarly, the type of induction therapy or the timing of therapy did sequent therapies. The choice of therapy in relapsed or refractory MCL
not predict OS in 118 patients with MCL treated with HDT/ASCT depends on the upfront chemotherapy regimen, outcomes with initial
in another study. The use of R-Hyper-CVAD, compared with therapy, performance status, comorbidities, and desired goals. Several
R-CHOP-like therapy, improved PFS for the entire MCL cohort options are available including ibrutinib as well as bortezomib, benda-
41
(p = .01) but not among transplant recipients (p = .26). These mustine, lenalidomide, or temsirolimus, frequently combined with
studies indicate that intensive induction therapy such as R-Hyper- rituximab (Table 81.3). Ibrutinib received an accelerated approval in
CVAD may be preferred to R-CHOP among patients who are not November 2013 based on a phase II study of relapsed or refractory
planned to undergo HDT/ASCT consolidation; however, the choice MCL (n = 111), which demonstrated an overall response rate of 68%
43
of initial therapy may not make a significant difference in OS of with an OS of 58% at 18 months. Given its oral route of administra-
patients who are scheduled to undergo HDT/ASCT. tion and tolerability, ibrutinib is increasingly utilized as the preferred
The use of rituximab during or after HDT/ASCT and incorpora- option in relapsed or refractory MCL. However, other regimens appear
tion of bortezomib to induction or conditioning regimen have to be equally efficacious and may be used as an alternative or as
demonstrated promising results. In a study, the use of pre-emptive third-line options. Patients who have not undergone upfront HDT/
2
rituximab therapy (375 mg/m weekly for 4 weeks) for molecular ASCT may benefit from HDT/ASCT after salvage therapy. In one
relapse after upfront HDT/ASCT resulted in re-induction of molecu- study, the use of HDT/ASCT demonstrated a median EFS and OS of
44
lar remission in 92%, and median molecular and clinical relapse-free 36% and 65%, respectively. In this setting, the use of R-Hyper-
42
survivals of 1.5 and 3.7 years, respectively. Rituximab maintenance CVAD salvage therapy prior to HDT/ASCT may result in lower
45
after R-Hyper-CVAD or upfront HDT/ASCT may also improve relapse rates but similar PFS and OS. Another study demonstrated a
131
PFS. The use of rituximab frequently increases the risk of neutropenia promising result with I-tositumomab prior to HDT/ASCT with a
and hypogammaglobulinemia; however, this may not necessarily complete response rate of 91% and 3-year PFS and OS of 61% and
translate to a substantially increased risk of major infections. 93%, respectively, highlighting the radiosensitive nature of MCL. 46
Central Nervous System Prophylaxis Allogeneic Stem Cell Transplantation
The incidence of CNS involvement at diagnosis and the role of Although allogeneic stem cell transplantation (alloSCT) is potentially
primary CNS prophylaxis in MCL are controversial. In relapsed curative, lack of large-scale randomized trials and high transplant-
MCL, CNS involvement is noted in up to 5–20% of patients, par- related mortality (TRM) has limited enthusiasm toward the use of
ticularly with blastoid variant, and portends a poor prognosis. CNS- alloSCT in MCL. Nonetheless, alloSCT should be considered in
penetrating agents such as high-dose methotrexate and cytarabine, young and fit patients with MCL with multiple relapsed disease or
with or without intrathecal chemotherapy, may provide sufficient ASCT failure. The outcomes of alloSCT have been compared with
CNS prophylaxis in patients undergoing intensive therapies. In those of ASCT in a few studies. In retrospective studies, alloSCT,
other patients with high-risk features such as blastoid morphology compared with ASCT, is associated with higher TRM, lower relapse,
or presence of neurologic symptoms, some centers perform lumbar and similar OS. In a study of 97 patients with MCL, the alloSCT
puncture and utilize intrathecal methotrexate or high-dose systemic group, compared with the ASCT group, was younger, more heavily
methotrexate. pretreated, and less likely to be in first complete remission. AlloSCT