Page 1465 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1465
1302 Part VII Hematologic Malignancies
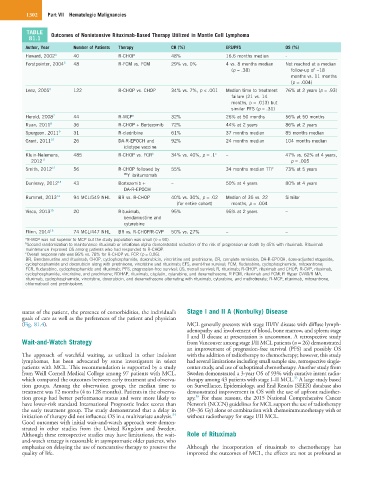
TABLE Outcomes of Nonintensive Rituximab-Based Therapy Utilized in Mantle Cell Lymphoma
81.1
Author, Year Number of Patients Therapy CR (%) EFS/PFS OS (%)
Howard, 2002 4 40 R-CHOP 48% 16.6 months median -
Forstpointer, 2004 5 48 R-FCM vs. FCM 29% vs. 0% 4 vs. 8 months median Not reached at a median
(p = .38) follow-up of ~18
months vs. 11 months
(p = .004)
Lenz, 2005 6 122 R-CHOP vs. CHOP 34% vs. 7%, p < .001 Median time to treatment 76% at 2 years (p = .93)
failure (21 vs. 14
months, p = .013) but
similar PFS (p = .31)
Herold, 2008 7 44 R-MCP a 32% 26% at 50 months 56% at 50 months
Ruan, 2011 8 36 R-CHOP + Bortezomib 72% 44% at 2 years 86% at 2 years
Spurgeon, 2011 9 31 R-cladribine 61% 37 months median 85 months median
Grant, 2011 10 26 DA-R-EPOCH and 92% 24 months median 104 months median
idiotype vaccine
Kluin-Nelemans, 485 R-CHOP vs. FCR b 34% vs. 40%, p = .1 c – 47% vs. 62% at 4 years,
2012 11 p = .005
Smith, 2012 12 56 R-CHOP followed by 55% 34 months median TTF 73% at 5 years
90 Y ibritumomab
Dunleavy, 2012 13 43 Bortezomib + – 50% at 4 years 80% at 4 years
DA-R-EPOCH
Rummel, 2013 14 94 MCL/549 NHL BR vs. R-CHOP 40% vs. 30%, p = .02 Median of 35 vs. 22 Similar
(for entire cohort) months, p = .004
Visco, 2013 15 20 Rituximab, 95% 95% at 2 years –
bendamustine and
cytarabine
Flinn, 2014 16 74 MCL/447 NHL BR vs. R-CHOP/R-CVP 50% vs. 27% – –
a R-MCP was not superior to MCP but the study population was small (n = 90).
b Second randomization to maintenance rituximab or interferon alpha demonstrated reduction of the risk of progression or death by 45% with rituximab. Rituximab
maintenance improved OS among patients who had responded to R-CHOP.
c Overall response rate was 86% vs. 78% for R-CHOP vs. FCR (p = 0.06).
BR, Bendamustine and rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; CR, complete remission; DA-R-EPOCH, dose-adjusted etoposide,
cyclophosphamide and doxorubicin along with prednisone, vincristine and rituximab; EFS, event-free survival; FCM, fludarabine, cyclophosphamide, mitoxantrone;
FCR, fludarabine, cyclophosphamide and rituximab; PFS, progression-free survival; OS, overall survival; R, rituximab; R-CHOP, rituximab and CHOP; R-CVP, rituximab,
cyclophosphamide, vincristine, and prednisone; RDHAP, rituximab, cisplatin, cytarabine, and dexamethasone; R-FCM, rituximab and FCM; R-Hyper-CVAD/R-MA,
rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with rituximab, cytarabine, and methotrexate; R-MCP, rituximab, mitoxantrone,
chlormabucil and prednisolone.
status of the patient, the presence of comorbidities, the individual’s Stage I and II A (Nonbulky) Disease
goals of care as well as the preferences of the patient and physician
(Fig. 81.4). MCL generally presents with stage III/IV disease with diffuse lymph-
adenopathy and involvement of blood, bone marrow, and spleen; stage
I and II disease at presentation is uncommon. A retrospective study
Wait-and-Watch Strategy from Vancouver among stage I/II MCL patients (n = 26) demonstrated
an improvement of progression-free survival (PFS) and possibly OS
The approach of watchful waiting, as utilized in other indolent with the addition of radiotherapy to chemotherapy; however, this study
lymphomas, has been advocated by some investigators in select had several limitations including small sample size, retrospective single-
patients with MCL. This recommendation is supported by a study center study, and use of suboptimal chemotherapy. Another study from
from Weill Cornell Medical College among 97 patients with MCL, Sweden demonstrated a 3-year OS of 93% with curative intent radio-
35
which compared the outcomes between early treatment and observa- therapy among 43 patients with stage I–II MCL. A large study based
tion groups. Among the observation group, the median time to on Surveillance, Epidemiology, and End Results (SEER) database also
treatment was 12 months (4 to 128 months). Patients in the observa- demonstrated improvement in OS with the use of upfront radiother-
36
tion group had better performance status and were more likely to apy. For these reasons, the 2015 National Comprehensive Cancer
have lower-risk standard International Prognostic Index scores than Network (NCCN) guidelines for MCL support the use of radiotherapy
the early treatment group. The study demonstrated that a delay in (30–36 Gy) alone or combination with chemoimmunotherapy with or
34
initiation of therapy did not influence OS in a multivariate analysis. without radiotherapy for stage I/II MCL.
Good outcomes with initial wait-and-watch approach were demon-
strated in other studies from the United Kingdom and Sweden.
Although these retrospective studies may have limitations, the wait- Role of Rituximab
and-watch strategy is reasonable in asymptomatic older patients, who
emphasize on delaying the use of noncurative therapy to preserve the Although the incorporation of rituximab to chemotherapy has
quality of life. improved the outcomes of MCL, the effects are not as profound as