Page 1519 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1519
1346 Part VII Hematologic Malignancies
signature associated with a more favorable outcome. Recurrent gains proliferation, or cytokine signaling. A low frequency of recurrent
of chromosomes 3q, 5q, and 21 have been noted in AITL, although mutations has also been observed in genes that impact important
the genes affected by these abnormalities remain unknown. Muta- T-cell functions such as TCR signaling (CD28, FYN).
tions of genes involved in epigenetic processes have been observed in
AITL more frequently than in the other PTCLs. Inactivating TET2
mutations are observed in 33%–76% of AITL and 38% of PTCL- Anaplastic Large-Cell Lymphoma, ALK-Positive
NOS, especially in cases expressing T FH markers, suggesting a biologic
relationship between AITL and a subset of PTCL-NOS exhibiting ALCL, ALK+ represents one of the few subtypes of PTCL defined
the T FH phenotype. Similar to PTCL-NOS, DNMT3A mutations are by recurrent chromosomal translocations. These involve the ALK
also detected in AITL, with a significant fraction of cases (73%) also gene located on chromosome 2p23, with the nucleophosmin gene
harboring TET2 mutations, suggesting oncogenic cooperation and (NPM), on 5q35, being the most common translocation partner
epigenetic deregulation in disease pathogenesis. Recent studies have (55%–85% of cases), resulting in t(2;5)(p23;q35); variant transloca-
shown IDH2 mutations at the R172 residue to occur exclusively in tions involving ALK and other partner genes are detected in the
AITL (20%–45% of cases), with the majority of cases also harboring remaining cases. The translocation t(2;5)(p23;q35) results in the
TET2 mutations. However, the prognostic implications of these fusion protein NPM-ALK, which leads to constitutive activation of
alterations, if any, are unclear at present. Unlike other neoplasms, the ALK tyrosine kinase and alterations in downstream signaling,
IDH1 mutations have not been detected in this entity. More recent metabolic, and prosurvival pathways, among others. These include
whole-exome and -genome sequencing analyses have revealed recur- the Janus kinase 3 (JAK3)/signal transducer and activator of tran-
rent RHOA G17V mutations in 53%–68% of AITL, with concomi- scription (STAT)3, phosphatidylinositol 3-kinase (PI3K)/protein
tant TET2 mutations being observed in 65%–100% of cases. The kinase (AKT)/mammalian target of rapamycin (mTOR), and the
RHOA G17V mutation interferes with RHOA signaling, possibly by phospholipase C-γ (PLC-γ)-mediated RAS-extracellular signal-
inhibiting wild-type RHOA function, which could alter cell motility, regulated kinase (ERK) pathways. Activation of Notch1 signaling has
also been reported. Overexpression of MYC is noted in a significant
number of cases and secondary MYC translocations have been associ-
ated with aggressive behavior. The cell of origin of ALCL, ALK+ is
unclear at present. It is speculated that different T-cell subsets could
acquire a cytotoxic phenotype as a consequence of cellular reprogram-
ming due to genetic or epigenetic aberrations, and some tumors show
activation of a Th17 differentiation program and increased expression
of NF-κB target genes.
Variant translocations involving ALK and other partner genes can
be suspected based on the pattern of immunohistochemical staining
for ALK (cytoplasmic, membranous, or nucleolar instead of the
nuclear and cytoplasmic). Pathologic consequences and molecular
alterations in signaling pathways as a consequence of variant ALK
translocations are not completely understood yet, but partial overlap
with gene expression profiles of NPM-ALK has been described.
While differences in gene expression profiles between certain
morphologic subtypes of ALCL, ALK+ have been reported, array
CGH analysis of NPM-ALK and variant ALK translocations have
revealed similar recurrent secondary genetic abnormalities including
gains at 17p and losses at 4q and 11q.
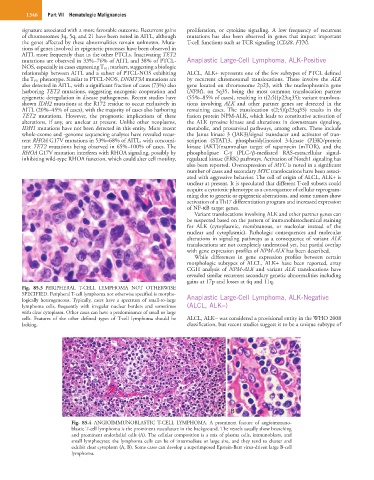
Fig. 85.3 PERIPHERAL T-CELL LYMPHOMA NOT OTHERWISE
SPECIFIED. Peripheral T-cell lymphoma not otherwise specified is morpho- Anaplastic Large-Cell Lymphoma, ALK-Negative
logically heterogeneous. Typically, cases have a spectrum of small-to-large
lymphoma cells, frequently with irregular nuclear borders and sometimes (ALCL, ALK−)
with clear cytoplasm. Other cases can have a predominance of small or large
cells. Features of the other defined types of T-cell lymphoma should be ALCL, ALK− was considered a provisional entity in the WHO 2008
lacking. classification, but recent studies suggest it to be a unique subtype of
A A B
Fig. 85.4 ANGIOIMMUNOBLASTIC T-CELL LYMPHOMA. A prominent feature of angioimmuno-
blastic T-cell lymphoma is the prominent vasculature in the background. The vessels usually show branching
and prominent endothelial cells (A). The cellular composition is a mix of plasma cells, immunoblasts, and
small lymphocytes; the lymphoma cells can be of intermediate or large size, and they tend to cluster and
exhibit clear cytoplasm (A, B). Some cases can develop a superimposed Epstein-Barr virus-driven large B-cell
lymphoma.