Page 1620 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1620
Chapter 88 Immunoglobulin Light Chain Amyloidosis (Primary Amyloidosis) 1441
1.0 response. Median survival was not reached for patients who achieved
0.9 a complete response, whereas it was 107 months for those with partial
remission and 32 months for nonresponders (Fig. 88.10). There is a
0.8 significant impact of pretreatment NT-proBNP levels on outcome.
0.7 We do not use chemomobilization to obtain stem cells. The low
number of plasma cells in the bone marrow allows growth factor–only
0.6
Surviving 0.5 mobilization to achieve the requisite goal. Plerixafor is not routinely
used to mobilize stem cells; it is used only in patients who do not
achieve a peripheral blood CD34 count >10/µL. We also do not
0.4
0.3 routinely use granulocyte colony-stimulating factor to support
engraftment following transplant, owing to fluid retention that is
0.2 seen, and we have observed no increase in bacteremia or hospital days
0.1 after ceasing use of posttransplant growth factor support. Among
amyloid patients, approximately 30% will complete the procedure
0.0 without requiring hospitalization. Adjuvant therapy has been used in
0 50 100 patients who are the recipients of stem cell transplants. Both
Survival months
thalidomide-dexamethasone and bortezomib-dexamethasone has
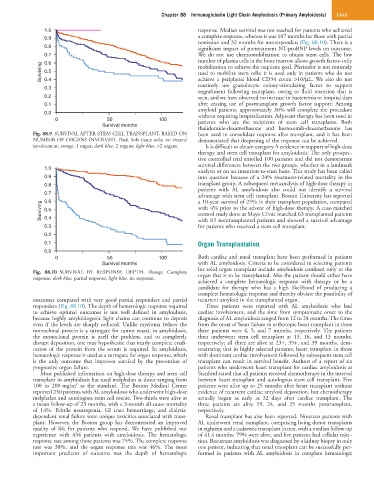
Fig. 88.9 SURVIVAL AFTER STEM CELL TRANSPLANT, BASED ON been used to consolidate response after transplant, and it has been
NUMBER OF ORGANS INVOLVED. Pink, Soft tissue only, no visceral demonstrated that deepening of the response can be achieved.
involvement; orange, 1 organ; dark blue, 2 organs; light blue, >2 organs. It is difficult to obtain category A evidence in support of high-dose
therapy and stem cell transplant for amyloidosis. The only prospec-
tive controlled trial enrolled 100 patients and did not demonstrate
survival differences between the two groups, whether in a landmark
1.0
analysis or on an intention-to-treat basis. This study has been called
0.9 into question because of a 24% treatment-related mortality in the
0.8 transplant group. A subsequent metaanalysis of high-dose therapy in
patients with AL amyloidosis also could not identify a survival
0.7 advantage with stem cell transplant. Boston University has reported
a 10-year survival of 25% in their transplant population, compared
0.6
Surviving 0.5 with 4% prior to the advent of high-dose therapy. A case-matched
control study done at Mayo Clinic matched 63 transplanted patients
0.4
for patients who received a stem cell transplant.
0.3 with 63 nontransplanted patients and showed a survival advantage
0.2
0.1 Organ Transplantation
0.0
0 50 100 Both cardiac and renal transplant have been performed in patients
Survival months with AL amyloidosis. Criteria to be considered in selecting patients
for solid organ transplant include amyloidosis confined only to the
Fig. 88.10 SURVIVAL BY RESPONSE DEPTH. Orange, Complete organ that is to be transplanted. Also the patient should either have
response; dark blue, partial response; light blue, no response.
achieved a complete hematologic response with therapy or be a
candidate for therapy who has a high likelihood of producing a
complete hematologic response and thereby obviate the possibility of
outcomes compared with very good partial responders and partial recurrent amyloid in the transplanted organ.
responders (Fig. 88.10). The depth of hematologic response required Three patients were reported with AL amyloidosis who had
to achieve optimal outcomes is not well defined in amyloidosis, cardiac involvement, and the time from symptomatic onset to the
because highly amyloidogenic light chains can continue to deposit diagnosis of AL amyloidosis ranged from 12 to 24 months. The times
even if the levels are sharply reduced. Unlike myeloma (where the from the onset of heart failure to orthotopic heart transplant in these
monoclonal protein is a surrogate for tumor mass), in amyloidosis, three patients were 4, 5, and 7 months, respectively. The patients
the monoclonal protein is itself the problem, and to completely then underwent stem cell transplant at 13, 16, and 13 months,
disrupt deposition, one may hypothesize that nearly complete eradi- respectively; all three are alive at 23+, 35+, and 39 months, dem-
cation of the protein from the serum is required. In amyloidosis, onstrating that in highly selected patients, heart transplant in those
hematologic response is used as a surrogate for organ response, which with dominant cardiac involvement followed by subsequent stem cell
is the only outcome that improves survival by the prevention of transplant can result in survival benefit. Authors of a report of six
progressive organ failure. patients who underwent heart transplant for cardiac amyloidosis at
Most published information on high-dose therapy and stem cell Stanford stated that all patients received chemotherapy in the interval
transplant in amyloidosis has used melphalan at doses ranging from between heart transplant and autologous stem cell transplant. Five
2
100 to 200 mg/m as the standard. The Boston Medical Center patients were alive up to 25 months after heart transplant without
reported 250 patients with AL amyloidosis who underwent high-dose evidence of recurrent cardiac amyloid deposition, but chemotherapy
melphalan and autologous stem cell rescue. Two-thirds were alive at actually began as early as 32 days after cardiac transplant. The
a mean follow-up of 23 months, with a 3-month all-cause mortality three patients are alive 19, 24, and 25 months posttransplant,
of 14%. Febrile neutropenia, GI tract hemorrhage, and dialysis- respectively.
dependent renal failure were unique toxicities associated with trans- Renal transplant has also been reported. Nineteen patients with
plant. However, the Boston group has demonstrated an improved AL underwent renal transplant, comprising living donor transplants
quality of life for patients who respond. We have published our in eighteen and a cadaveric transplant in one, with a median follow-up
experience with 434 patients with amyloidosis. The hematologic of 41.4 months; 79% were alive, and five patients had cellular rejec-
response rate among those patients was 75%. The complete response tion. Recurrent amyloidosis was diagnosed by a kidney biopsy in only
rate was 38%, and the organ response rate was 46%. The most one patient, indicating that renal transplant can be successfully per-
important predictor of outcome was the depth of hematologic formed in patients with AL amyloidosis in complete hematologic