Page 1629 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1629
1450 Part VIII Comprehensive Care of Patients with Hematologic Malignancies
Hemoglobin Gene Variants 100
Patients with chronic hemolytic states may develop bilirubin PMN <100/mm 3
gallstones, which can serve as a nidus for infection. Defects in cell- 80
mediated immunity have been described in patients with thalassemia.
Patients with sickle cell disease have an increased susceptibility to PMN <1000/mm 3
7
bacterial infections. Defective alternative complement pathway 60
function, especially in conjunction with asplenia, contributes to Percent of patients with serious infection
the propensity to bacterial infection. Splenic involution results in 40
depressed synthesis of alternate pathway factor(s) of complement
and decreased phagocytic clearance of bacteria. Phagocytosis of
Streptococcus pneumoniae is abnormal, in part because of an inability 20
to use the alternate pathway for C3 fixation as a means of opsoniza-
tion. An increased risk for Salmonella infection appears to be unique
to the sickle cell population. Suppurative arthritis can occur after 0
repeated episodes of hemarthrosis among patients with sickle cell 0 2 4 6 8 10 12 14
disease.
Weeks
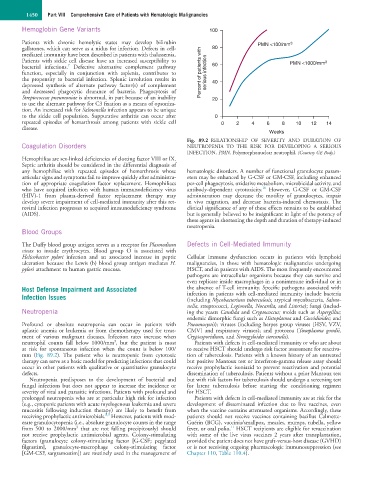
Fig. 89.2 RELATIONSHIP OF SEVERITY AND DURATION OF
Coagulation Disorders NEUTROPENIA TO THE RISK FOR DEVELOPING A SERIOUS
INFECTION. PMN, Polymorphonuclear neutrophil. (Courtesy GE Body.)
Hemophilias are sex-linked deficiencies of clotting factor VIII or IX.
Septic arthritis should be considered in the differential diagnosis of
any hemophiliac with repeated episodes of hemarthrosis whose hematologic disorders. A number of functional granulocyte param-
articular signs and symptoms fail to improve quickly after administra- eters may be enhanced by G-CSF or GM-CSF, including enhanced
tion of appropriate coagulation factor replacement. Hemophiliacs per-cell phagocytosis, oxidative metabolism, microbicidal activity, and
10
who have acquired infection with human immunodeficiency virus antibody-dependent cytotoxicity. However, G-CSF or GM-CSF
(HIV)-1 from plasma-derived factor replacement therapy may administration may decrease the motility of granulocytes, impair
develop severe impairment of cell-mediated immunity after this ret- in vivo migration, and decrease bacteria-induced chemotaxis. The
roviral infection progresses to acquired immunodeficiency syndrome clinical significance of any of these effects remains to be established
(AIDS). but is generally believed to be insignificant in light of the potency of
these agents in shortening the depth and duration of therapy-induced
neutropenia.
Blood Groups
The Duffy blood group antigen serves as a receptor for Plasmodium Defects in Cell-Mediated Immunity
vivax to invade erythrocytes. Blood group O is associated with
Helicobacter pylori infection and an associated increase in peptic Cellular immune dysfunction occurs in patients with lymphoid
ulceration because the Lewis (b) blood group antigen mediates H. malignancies, in those with hematologic malignancies undergoing
pylori attachment to human gastric mucosa. HSCT, and in patients with AIDS. The most frequently encountered
pathogens are intracellular organisms because they can survive and
even replicate inside macrophages in a nonimmune individual or in
Host Defense Impairment and Associated the absence of T-cell immunity. Specific pathogens associated with
Infection Issues infection in patients with cell-mediated immunity include bacteria
(including Mycobacterium tuberculosis, atypical mycobacteria, Salmo-
nella, streptococci, Legionella, Nocardia, and Listeria); fungi (includ-
Neutropenia ing the yeasts Candida and Cryptococcus; molds such as Aspergillus;
endemic dimorphic fungi such as Histoplasma and Coccidioides; and
Profound or absolute neutropenia can occur in patients with Pneumocystis); viruses (including herpes group viruses [HSV, VZV,
aplastic anemia or leukemia or from chemotherapy used for treat- CMV] and respiratory viruses); and protozoa (Toxoplasma gondii,
ment of various malignant diseases. Infection rates increase when Cryptosporidium, and Strongyloides stercoralis).
3
neutrophil counts fall below 1000/mm , but the patient is most Patients with defects in cell-mediated immunity or who are about
at risk for spontaneous infection when the count is below 100/ to receive HSCT should undergo risk factor assessment for reactiva-
mm (Fig. 89.2). The patient who is neutropenic from cytotoxic tion of tuberculosis. Patients with a known history of an untreated
therapy can serve as a basic model for predicting infections that could but positive Mantoux test or interferon-gamma release assay should
occur in other patients with qualitative or quantitative granulocyte receive prophylactic isoniazid to prevent reactivation and potential
defects. dissemination of tuberculosis. Patients without a prior Mantoux test
Neutropenia predisposes to the development of bacterial and but with risk factors for tuberculosis should undergo a screening test
fungal infections but does not appear to increase the incidence or for latent tuberculosis before starting the conditioning regimen
severity of viral and parasitic infections. Patients with profound and for HSCT.
prolonged neutropenia who are at particular high risk for infection Patients with defects in cell-mediated immunity are at risk for the
(e.g., cytopenic patients with acute myelogenous leukemia and severe development of disseminated infection due to live vaccines, even
mucositis following induction therapy) are likely to benefit from when the vaccine contains attenuated organisms. Accordingly, these
8,9
receiving prophylactic antimicrobials. However, patients with mod- patients should not receive vaccines containing bacillus Calmette-
erate granulocytopenia (i.e., absolute granulocyte counts in the range Guérin (BCG), vaccinia/smallpox, measles, mumps, rubella, yellow
11
3
from 500 to 2000/mm that are not falling precipitously) should fever, or oral polio. HSCT recipients are eligible for revaccination
not receive prophylactic antimicrobial agents. Colony-stimulating with some of the live virus vaccines 2 years after transplantation,
factors (granulocyte colony-stimulating factor [G-CSF; pegylated provided the patient does not have graft-versus-host disease (GVHD)
filgrastim], granulocyte-macrophage colony-stimulating factor or is not receiving ongoing pharmacologic immunosuppression (see
[GM-CSF, sargramostim]) are routinely used in the management of Chapter 110, Table 110.4).