Page 1997 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1997
Chapter 117 Transfusion Therapy for Coagulation Factor Deficiencies 1771
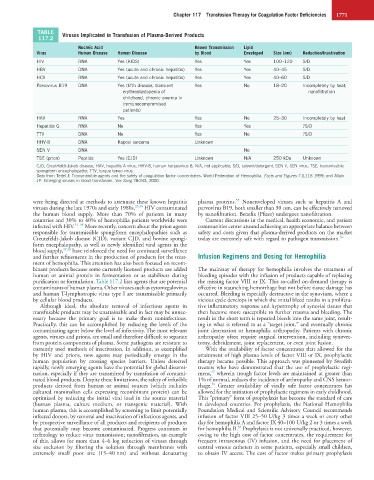
TABLE Viruses Implicated in Transfusion of Plasma-Derived Products
117.2
Nucleic Acid Known Transmission Lipid
Virus Human Disease Human Disease by Blood Enveloped Size (nm) Reduction/Inactivation
HIV RNA Yes (AIDS) Yes Yes 100–120 S/D
HBV DNA Yes (acute and chronic hepatitis) Yes Yes 40–45 S/D
HCV RNA Yes (acute and chronic hepatitis) Yes Yes 40–60 S/D
Parvovirus B19 DNA Yes (fifth disease, transient Yes No 18–20 Incompletely by heat;
erythroblastopenia of nanofiltration
childhood, chronic anemia in
immunocompromised
patients)
HAV RNA Yes Yes No 25–30 Incompletely by heat
Hepatitis G RNA No Yes Yes ?S/D
TTV DNA No Yes No ?S/D
HHV-8 DNA Kaposi sarcoma Unknown
SEN V DNA No
TSE (prion) Peptide Yes (CJD) Unknown N/A 250 kDa Unknown
CJD, Creutzfeldt-Jakob disease; HAV, hepatitis A virus; HHV-8, human herpesvirus 8; N/A, not applicable; S/D, solvent/detergent; SEN V, SEN virus; TSE, transmissible
spongiform encephalopathy; TTV, torque teneo virus.
Data from Teitel J: Transmissible agents and the safety of coagulation factor concentrates. World Federation of Hemophilia. Facts and Figures 7:1,118 1999; and Allain
JP: Emerging viruses in blood transfusion. Vox Sang 78:243, 2000.
55
were being directed at methods to attenuate these known hepatitis plasma proteins. Nonenveloped viruses such as hepatitis A and
viruses during the late 1970s and early 1980s, 23,36 HIV contaminated parvovirus B19, both smaller than 30 nm, can be effectively removed
the human blood supply. More than 70% of patients in many by nanofiltration. Benefix (Pfizer) undergoes nanofiltration.
countries and 30% to 40% of hemophilia patients worldwide were Current discussions in the medical, health economic, and patient
infected with HIV. 51–54 More recently, concern about the prion agents communities center around achieving an appropriate balance between
responsible for transmissible spongiform encephalopathies such as safety and costs given that plasma-derived products on the market
Creutzfeldt-Jakob disease (CJD), variant CJD, and bovine spongi- today are extremely safe with regard to pathogen transmission. 56
form encephalopathy, as well as newly identified viral agents in the
blood supply, 47,48 have reinforced the need for continued surveillance
and further refinements in the production of products for the treat- Infusion Regimens and Dosing for Hemophilia
ment of hemophilia. This attention has also been focused on recom-
binant products because some currently licensed products use added The mainstay of therapy for hemophilia involves the treatment of
human or animal protein in fermentation or as stabilizers during bleeding episodes with the infusion of products capable of replacing
purification or formulation. Table 117.2 lists agents that are potential the missing factor VIII or IX. This so-called on-demand therapy is
contaminants of human plasma. Other viruses such as cytomegalovirus effective in staunching hemorrhage but not before tissue damage has
and human T-lymphotropic virus type I are transmissible primarily occurred. Bleeding is especially destructive in the synovium, where a
by cellular blood products. vicious cycle develops in which the initial bleed results in a prolifera-
Although ideal, the absolute removal of infectious agents in tive inflammatory response and hypertrophy of synovial tissues that
transfusable products may be unattainable and in fact may be unnec- then become more susceptible to further trauma and bleeding. The
essary because the primary goal is to make them noninfectious. result in the short term is repeated bleeds into the same joint, result-
Practically, this can be accomplished by reducing the levels of the ing in what is referred to as a “target joint,” and eventually chronic
contaminating agent below the level of infectivity. The most relevant joint destruction or hemophilic arthropathy. Patients with chronic
agents, viruses and prions, are small and therefore difficult to separate arthropathy often require surgical intervention, including synovec-
from protein components of plasma. Some pathogens are resistant to tomy, debridement, joint replacement, or even joint fusion.
currently used methods of inactivation. In addition, as exemplified With the availability of factor concentrates that allowed for the
by HIV and prions, new agents may periodically emerge in the attainment of high plasma levels of factor VIII or IX, prophylactic
human population by crossing species barriers. Unless detected therapy became possible. This approach was pioneered by Swedish
rapidly, newly emerging agents have the potential for global dissemi- treaters who have demonstrated that the use of prophylactic regi-
57
nation, especially if they are transmitted by transfusion of contami- mens, wherein trough factor levels are maintained at greater than
nated blood products. Despite these limitations, the safety of infusible 1% of normal, reduces the incidence of arthropathy and CNS hemor-
56
products derived from human or animal sources (which includes rhage. Greater availability of virally safe factor concentrates has
cultured mammalian cells expressing recombinant protein) can be allowed for the initiation of prophylactic regimens in early childhood.
optimized by reducing the initial viral load in the source material This “primary” form of prophylaxis has become the standard of care
(human plasma, culture medium, or transgenic material). With in developed countries. For prophylaxis, the National Hemophilia
human plasma, this is accomplished by screening to limit potentially Foundation Medical and Scientific Advisory Council recommends
infected donors, by removal and inactivation of infectious agents, and infusion of factor VIII 25–50 U/kg 3 times a week or every other
by prospective surveillance of all products and recipients of products day for hemophilia A and factor IX 40–100 U/kg 2 or 3 times a week
58
that potentially may become contaminated. Progress continues in for hemophilia B. Prophylaxis is not universally practiced, however,
technology to reduce virus transmission; nanofiltration, an example owing to the high cost of factor concentrates, the requirement for
of this, allows for more than 4–6 log reduction of viruses through frequent intravenous (IV) infusion, and the need for placement of
size exclusion by filtering the solution through membranes with central venous catheters in some patients, especially small children,
extremely small poor size (15–40 nm) and without denaturing to obtain IV access. The cost of factor makes primary prophylaxis