Page 1993 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1993
Chapter 116 Preparation of Plasma-Derived and Recombinant Human Plasma Proteins 1767
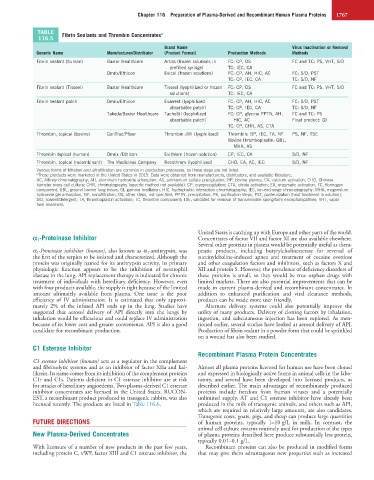
TABLE Fibrin Sealants and Thrombin Concentrates a
116.5
Brand Name Virus Inactivation or Removal
Generic Name Manufacturer/Distributor (Product Format) Production Methods Methods
Fibrin sealant (human) Baxter Healthcare Artiss (frozen solutions in FC: CP, OS FC and TC: PS, VHT, S/D
prefilled syringe) TC: IEC, CA
Omrix/Ethicon Evicel (frozen solutions) FC: CP, AH, HIC, AC FC: S/D, PST
TC: CP, IEC, CA TC: S/D, NF
Fibrin sealant (Tisseel) Baxter Healthcare Tisseel (lyophilized or frozen FC: CP, OS FC and TC: PS, VHT, S/D
solutions) TC: IEC, CA
Fibrin sealant patch Omrix/Ethicon Evarrest (lyophilized FC: CP, AH, HIC, AC FC: S/D, PST
absorbable patch) TC: CP, IEC, CA TC: S/D, NF
Takeda/Baxter Healthcare TachoSil (lyophilized FC: CP, glycine PPTN, AH, FC and TC: PS
absorbable patch) HIC, AC Final product: GI
TC: CP, CHR, AS, CTA
Thrombin, topical (bovine) GenTrac/Pfizer Thrombin-JMI (lyophilized) Thrombin: BP, IEC, TA, NF PS, NF, TSE
Bovine thromboplastin: GBL,
MHA, AS
Thrombin topical (human) Omrix /Ethicon Evithrom (frozen solution) CP, IEC, CA S/D, NF
Thrombin, topical (recombinant) The Medicines Company Recothrom (lyophilized) CHO, EA, AC, IEC S/D, NF
Various forms of filtration and ultrafiltration are common in production processes, so those steps are not listed.
a These products were marketed in the United States in 2015. Data were obtained from manufacturers, distributors, and available literature.
AC, Affinity chromatography; AH, aluminum hydroxide adsorption; AS, ammonium sulfate precipitation; BP, bovine plasma; CA, calcium activation; CHO, Chinese
hamster ovary cell culture; CHR, chromatography (specific method not available); CP, cryoprecipitation; CTA, citrate activation; EA, enzymatic activation; FC, fibrinogen
component; GBL, ground bovine lung tissue; GI, gamma irradiation; HIC, hydrophobic interaction chromatography; IEC, ion-exchange chromatography; MHA, magnesium
hydroxide gel adsorption; NF, nanofiltration; OS, other steps, not specified; PPTN, precipitation; PS, purification steps; PST, pasteurization (heat treatment in solution);
S/D, solvent/detergent; TA, thromboplastin activation; TC, thrombin component; TSE, validated for removal of transmissible spongiform encephalopathies; VHT, vapor
heat treatment.
United States is catching up with Europe and other parts of the world.
α 1-Proteinase Inhibitor Concentrates of factor VII and factor XI are also available elsewhere.
Several other proteins in plasma would be potentially useful as thera-
α 1 -Proteinase inhibitor (human), also known as α 1 -antitrypsin, was peutic products, including butyrylcholinesterase for reversal of
the first of the serpins to be isolated and characterized. Although the succinylcholine-induced apnea and treatment of cocaine overdose
protein was originally named for its antitrypsin activity, its primary and other coagulation factors and inhibitors, such as factors X and
physiologic function appears to be the inhibition of neutrophil XII and protein S. However, the prevalence of deficiency disorders of
elastase in the lung. API replacement therapy is indicated for chronic these proteins is small, so they would be true orphan drugs with
treatment of individuals with hereditary deficiency. However, even limited markets. There are also potential improvements that can be
with four products available, the supply is tight because of the limited made to current plasma-derived and recombinant concentrates. In
amount ultimately available from plasma. One issue is the poor addition to enhanced purification and viral clearance methods,
efficiency of IV administration. It is estimated that only approxi- products can be made more user friendly.
mately 2% of the infused API ends up in the lung. Studies have Alternate delivery systems could also potentially improve the
suggested that aerosol delivery of API directly into the lungs by utility of many products. Delivery of clotting factors by inhalation,
inhalation would be efficacious and could replace IV administration ingestion, and subcutaneous injection has been explored. As men-
because of its lower cost and greater convenience. API is also a good tioned earlier, several studies have looked at aerosol delivery of API.
candidate for recombinant production. Production of fibrin sealant in a powder form that could be sprinkled
on a wound has also been studied.
C1 Esterase Inhibitor
Recombinant Plasma Protein Concentrates
C1 esterase inhibitor (human) acts as a regulator in the complement
and fibrinolytic systems and as an inhibitor of factor XIIa and kal- Almost all plasma proteins licensed for human use have been cloned
likrein. Its name comes from its inhibition of the complement proteins and expressed in biologically active forms in animal cells in the labo-
C1r and C1s. Patients deficient in C1 esterase inhibitor are at risk ratory, and several have been developed into licensed products, as
for attacks of hereditary angioedema. Two plasma-derived C1 esterase described earlier. The main advantages of recombinantly produced
inhibitor concentrates are licensed in the United States. RUCON- proteins include freedom from human viruses and a potentially
EST, a recombinant product produced in transgenic rabbits, was also unlimited supply. AT and C1 esterase inhibitor have already been
licensed recently. The products are listed in Table 116.6. produced in the milk of transgenic animals, and others such as API,
which are required in relatively large amounts, are also candidates.
Transgenic cows, goats, pigs, and sheep can produce large quantities
FUTURE DIRECTIONS of human proteins, typically 1–10 g/L in milk. In contrast, the
animal cell culture systems routinely used for production of the types
New Plasma-Derived Concentrates of plasma proteins described here produce substantially less protein,
typically 0.01–0.1 g/L.
With licensure of a number of new products in the past few years, Recombinant proteins can also be produced in modified forms
including protein C, vWF, factor XIII and C1 esterase inhibitor, the that may give them advantageous new properties such as increased