Page 2064 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2064
Chapter 122 Overview of Hemostasis and Thrombosis 1833
Plaque disruption
Tissue Factor Collagen vWF
Platelet adhesion and secretion
Aspirin X COX-1
ADP
TXA 2 Ticlopidine
Clopidogrel
X
Thrombin Prasugrel
Ticagrelor
Platelet recruitment
X
and activation
Vorapaxar
GPIIb/IIIa activation
Abciximab
X Eptifibatide
Tirofiban
Platelet aggregation
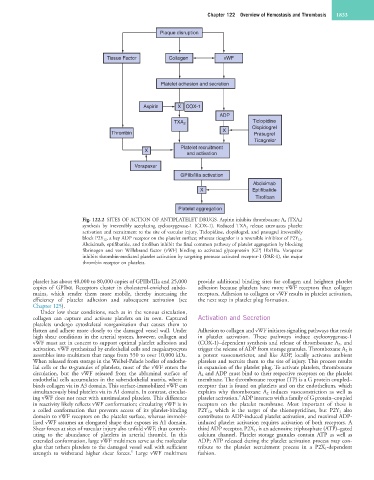
Fig. 122.2 SITES OF ACTION OF ANTIPLATELET DRUGS. Aspirin inhibits thromboxane A 2 (TXA 2)
synthesis by irreversibly acetylating cyclooxygenase-1 (COX-1). Reduced TXA 2 release attenuates platelet
activation and recruitment to the site of vascular injury. Ticlopidine, clopidogrel, and prasugrel irreversibly
block P2Y 12, a key ADP receptor on the platelet surface; whereas ticagrelor is a reversible inhibitor of P2Y 12.
Abciximab, eptifibatide, and tirofiban inhibit the final common pathway of platelet aggregation by blocking
fibrinogen and von Willebrand factor (vWF) binding to activated glycoprotein (GP) IIb/IIIa. Vorapaxar
inhibits thrombin-mediated platelet activation by targeting protease activated receptor-1 (PAR-1), the major
thrombin receptor on platelets.
platelet has about 40,000 to 80,000 copies of GPIIb/IIIa and 25,000 provide additional binding sites for collagen and heighten platelet
copies of GPIbα. Receptors cluster in cholesterol-enriched subdo- adhesion because platelets have more vWF receptors than collagen
mains, which render them more mobile, thereby increasing the receptors. Adhesion to collagen or vWF results in platelet activation,
efficiency of platelet adhesion and subsequent activation (see the next step in platelet plug formation.
Chapter 125).
Under low shear conditions, such as in the venous circulation,
collagen can capture and activate platelets on its own. Captured Activation and Secretion
platelets undergo cytoskeletal reorganization that causes them to
flatten and adhere more closely to the damaged vessel wall. Under Adhesion to collagen and vWF initiates signaling pathways that result
high shear conditions in the arterial system, however, collagen and in platelet activation. These pathways induce cyclooxygenase-1
vWF must act in concert to support optimal platelet adhesion and (COX-1)–dependent synthesis and release of thromboxane A 2 , and
activation. vWF synthesized by endothelial cells and megakaryocytes trigger the release of ADP from storage granules. Thromboxane A 2 is
assembles into multimers that range from 550 to over 10,000 kDa. a potent vasoconstrictor, and like ADP, locally activates ambient
When released from storage in the Weibel-Palade bodies of endothe- platelets and recruits them to the site of injury. This process results
lial cells or the α-granules of platelets, most of the vWF enters the in expansion of the platelet plug. To activate platelets, thromboxane
circulation, but the vWF released from the abluminal surface of A 2 and ADP must bind to their respective receptors on the platelet
endothelial cells accumulates in the subendothelial matrix, where it membrane. The thromboxane receptor (TP) is a G protein coupled–
binds collagen via its A3 domain. This surface-immobilized vWF can receptor that is found on platelets and on the endothelium, which
simultaneously bind platelets via its A1 domain. In contrast, circulat- explains why thromboxane A 2 induces vasoconstriction as well as
9
ing vWF does not react with unstimulated platelets. This difference platelet activation. ADP interacts with a family of G protein–coupled
in reactivity likely reflects vWF conformation; circulating vWF is in receptors on the platelet membrane. Most important of these is
a coiled conformation that prevents access of its platelet-binding P2Y 12, which is the target of the thienopyridines, but P2Y 1 also
domain to vWF receptors on the platelet surface, whereas immobi- contributes to ADP-induced platelet activation, and maximal ADP-
lized vWF assumes an elongated shape that exposes its A1 domain. induced platelet activation requires activation of both receptors. A
Shear forces at sites of vascular injury also unfold vWF, thus contrib- third ADP receptor, P2X 1, is an adenosine triphosphate (ATP)–gated
uting to the abundance of platelets in arterial thrombi. In this calcium channel. Platelet storage granules contain ATP as well as
extended conformation, large vWF multimers serve as the molecular ADP; ATP released during the platelet activation process may con-
glue that tethers platelets to the damaged vessel wall with sufficient tribute to the platelet recruitment process in a P2X 1-dependent
8
strength to withstand higher shear forces. Large vWF multimers fashion.