Page 2065 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2065
1834 Part XII Hemostasis and Thrombosis
IIa
IIa
IIa LDPR
LDPRSFLLR RLLFS
C C
C C
Response
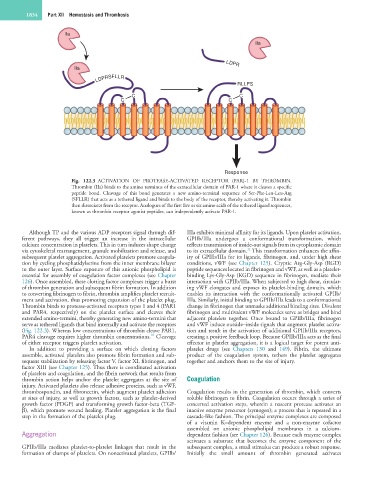
Fig. 122.3 ACTIVATION OF PROTEASE-ACTIVATED RECEPTOR (PAR)-1 BY THROMBIN.
Thrombin (IIa) binds to the amino terminus of the extracellular domain of PAR-1 where it cleaves a specific
peptide bond. Cleavage of this bond generates a new amino-terminal sequence of Ser-Phe-Leu-Leu-Arg
(SFLLR) that acts as a tethered ligand and binds to the body of the receptor, thereby activating it. Thrombin
then dissociates from the receptor. Analogues of the first five or six amino acids of the tethered ligand sequences,
known as thrombin receptor agonist peptides, can independently activate PAR-1.
Although TP and the various ADP receptors signal through dif- IIIa exhibits minimal affinity for its ligands. Upon platelet activation,
ferent pathways, they all trigger an increase in the intracellular GPIIb/IIIa undergoes a conformational transformation, which
calcium concentration in platelets. This in turn induces shape change reflects transmission of inside-out signals from its cytoplasmic domain
11
via cytoskeletal rearrangement, granule mobilization and release, and to its extracellular domain. This transformation enhances the affin-
subsequent platelet aggregation. Activated platelets promote coagula- ity of GPIIb/IIIa for its ligands, fibrinogen, and, under high shear
tion by cycling phosphatidylserine from the inner membrane bilayer conditions, vWF (see Chapter 125). Cryptic Arg-Gly-Asp (RGD)
to the outer layer. Surface exposure of this anionic phospholipid is peptide sequences located in fibrinogen and vWF, as well as a platelet-
essential for assembly of coagulation factor complexes (see Chapter binding Lys-Gly-Asp (KGD) sequence in fibrinogen, mediate their
126). Once assembled, these clotting factor complexes trigger a burst interaction with GPIIb/IIIa. When subjected to high shear, circulat-
of thrombin generation and subsequent fibrin formation. In addition ing vWF elongates and exposes its platelet-binding domain, which
to converting fibrinogen to fibrin, thrombin amplifies platelet recruit- enables its interaction with the conformationally activated GPIIb/
ment and activation, thus promoting expansion of the platelet plug. IIIa. Similarly, initial binding to GPIIb/IIIa leads to a conformational
Thrombin binds to protease-activated receptors types 1 and 4 (PAR1 change in fibrinogen that unmasks additional binding sites. Divalent
and PAR4, respectively) on the platelet surface and cleaves their fibrinogen and multivalent vWF molecules serve as bridges and bind
extended amino-termini, thereby generating new amino-termini that adjacent platelets together. Once bound to GPIIb/IIIa, fibrinogen
serve as tethered ligands that bind internally and activate the receptors and vWF induce outside–inside signals that augment platelet activa-
(Fig. 122.3). Whereas low concentrations of thrombin cleave PAR1, tion and result in the activation of additional GPIIb/IIIa receptors,
10
PAR4 cleavage requires higher thrombin concentrations. Cleavage creating a positive feedback loop. Because GPIIb/IIIa acts as the final
of either receptor triggers platelet activation. effector in platelet aggregation, it is a logical target for potent anti-
In addition to providing a surface on which clotting factors platelet drugs (see Chapters 130 and 149). Fibrin, the ultimate
assemble, activated platelets also promote fibrin formation and sub- product of the coagulation system, tethers the platelet aggregates
sequent stabilization by releasing factor V, factor XI, fibrinogen, and together and anchors them to the site of injury.
factor XIII (see Chapter 125). Thus there is coordinated activation
of platelets and coagulation, and the fibrin network that results from
thrombin action helps anchor the platelet aggregates at the site of Coagulation
injury. Activated platelets also release adhesive proteins, such as vWF,
thrombospondin, and fibronectin, which augment platelet adhesion Coagulation results in the generation of thrombin, which converts
at sites of injury, as well as growth factors, such as platelet-derived soluble fibrinogen to fibrin. Coagulation occurs through a series of
growth factor (PDGF) and transforming growth factor-beta (TGF- concerted activation steps, wherein a nascent protease activates an
β), which promote wound healing. Platelet aggregation is the final inactive enzyme precursor (zymogen); a process that is repeated in a
step in the formation of the platelet plug. cascade-like fashion. The principal enzyme complexes are composed
of a vitamin K–dependent enzyme and a non-enzyme cofactor
assembled on anionic phospholipid membranes in a calcium-
Aggregation dependent fashion (see Chapter 126). Because each enzyme complex
activates a substrate that becomes the enzyme component of the
GPIIb/IIIa mediates platelet-to-platelet linkages that result in the subsequent complex, a small stimulus can produce a robust response.
formation of clumps of platelets. On nonactivated platelets, GPIIb/ Initially the small amount of thrombin generated activates