Page 2125 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2125
1886 Part XII Hemostasis and Thrombosis
Injury Legend
Intrinsic pathway Extrinsic pathway Enzymes
Factor XII Exposed Inhibitors
Prekallikrein Circulating Tissue factor
HMWK Factor VIIa Anionic membrane Zymogens
“Surface” Ca 2+ Complexes
AT Factor X
FXIa Intrinsic tenase Extrinsic tenase Factor IX
TFPI
Factor IXa AT Factor VIIa
Factor VIIIa Tissue factor
Factor IX FIXa FIXa Factor X
Anionic membrane Anionic membrane
Ca 2+ Ca 2+
FXa Prothrombinase FXa
FXIa AT
TFPI
AT Factor Xa AT Fibrinogen
Factor II Factor Va
Anionic membrane
Activated platelets Ca 2+ Factor XIII
factor XI
FPA
Factor IIa
AT FIIa FIIa
AT
FIIa FPB
(FXIIa) FXIIIa
FVa i
Thrombin
FVIIIa i Thrombomodulin Crosslinked
APC TAFIa
Anionic membrane fibrin
Ca 2+
Protein Case
Protein C TAFI
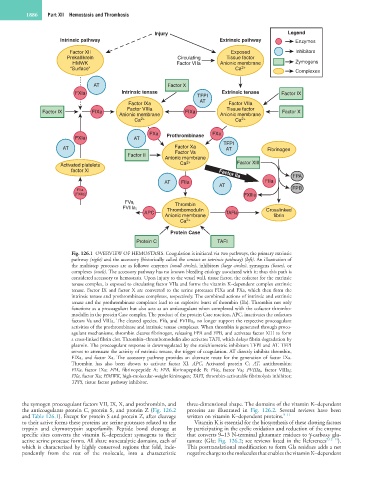
Fig. 126.1 OVERVIEW OF HEMOSTASIS. Coagulation is initiated via two pathways, the primary extrinsic
pathway (right) and the accessory (historically called the contact or intrinsic pathway) (left). An illustration of
the multistep processes are as follows: enzymes (small circles), inhibitors (large circles), zymogens (boxes), or
complexes (ovals). The accessory pathway has no known bleeding etiology associated with it; thus this path is
considered accessory to hemostasis. Upon injury to the vessel wall, tissue factor, the cofactor for the extrinsic
tenase complex, is exposed to circulating factor VIIa and forms the vitamin K–dependent complex extrinsic
tenase. Factor IX and factor X are converted to the serine proteases FIXa and FXa, which then form the
intrinsic tenase and prothrombinase complexes, respectively. The combined actions of intrinsic and extrinsic
tenase and the prothrombinase complexes lead to an explosive burst of thrombin (IIa). Thrombin not only
functions as a procoagulant but also acts as an anticoagulant when complexed with the cofactor thrombo-
modulin in the protein Case complex. The product of the protein Case reaction, APC, inactivates the cofactors
factors Va and VIIIa. The cleaved species, FVa i and FVIIIa i , no longer support the respective procoagulant
activities of the prothrombinase and intrinsic tenase complexes. When thrombin is generated through proco-
agulant mechanisms, thrombin cleaves fibrinogen, releasing FPA and FPB, and activates factor XIII to form
a cross-linked fibrin clot. Thrombin–thrombomodulin also activates TAFI, which delays fibrin degradation by
plasmin. The procoagulant response is downregulated by the stoichiometric inhibitors TFPI and AT. TFPI
serves to attenuate the activity of extrinsic tenase, the trigger of coagulation. AT directly inhibits thrombin,
FIXa, and factor Xa. The accessory pathway provides an alternate route for the generation of factor IXa.
Thrombin has also been shown to activate factor XI. APC, Activated protein C; AT, antithrombin;
FIXa, factor IXa; FPA, fibrinopeptide A; FPB, fibrinopeptide B; FVa i , factor Va i ; FVIIIa i , factor VIIIa i ;
FXa, factor Xa; HMWK, high-molecular-weight kininogen; TAFI, thrombin-activatable fibrinolysis inhibitor;
TFPI, tissue factor pathway inhibitor.
the zymogen procoagulant factors VII, IX, X, and prothrombin, and three-dimensional shape. The domains of the vitamin K–dependent
the anticoagulants protein C, protein S, and protein Z (Fig. 126.2 proteins are illustrated in Fig. 126.2. Several reviews have been
and Table 126.1). Except for protein S and protein Z, after cleavage written on vitamin K–dependent proteins. 9–11
to their active forms these proteins are serine proteases related to the Vitamin K is essential for the biosynthesis of these clotting factors
trypsin and chymotrypsin superfamily. Peptide bond cleavage at by participating in the cyclic oxidation and reduction of the enzyme
specific sites converts the vitamin K–dependent zymogens to their that converts 9–13 N-terminal glutamate residues to γ-carboxy glu-
active serine protease forms. All share noncatalytic domains, each of tamate (Gla; Fig. 126.2; see reviews listed in the References 9,12–14 ).
which is characterized by highly conserved regions that fold, inde- This posttranslational modification to form Gla residues adds a net
pendently from the rest of the molecule, into a characteristic negative charge to the molecules that enables the vitamin K–dependent