Page 2128 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2128
Chapter 126 Molecular Basis of Blood Coagulation 1889
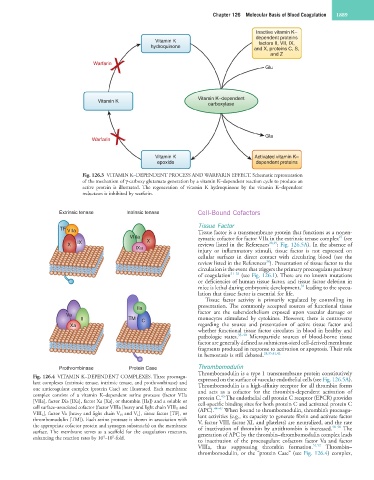
Inactive vitamin K–
dependent proteins
Vitamin K factors II, VII, IX,
hydroquinone
and X, proteins C, S,
and Z
Warfarin
Glu
Vitamin K–dependent
Vitamin K
carboxylase
Gla
Warfarin
Vitamin K Activated vitamin K–
epoxide dependent proteins
Fig. 126.3 VITAMIN K–DEPENDENT PROCESS AND WARFARIN EFFECT. Schematic representation
of the mechanism of γ-carboxy glutamate generation by a vitamin K–dependent reaction cycle to produce an
active protein is illustrated. The regeneration of vitamin K hydroquinone by the vitamin K–dependent
reductases is inhibited by warfarin.
Extrinsic tenase Intrinsic tenase Cell-Bound Cofactors
Tissue Factor
TF VIIa Tissue factor is a transmembrane protein that functions as a nonen-
VIIIa zymatic cofactor for factor VIIa in the extrinsic tenase complex (see
27
X IX X reviews listed in the References 28,29 ; Fig. 126.5A). In the absence of
IXa
injury or inflammatory stimuli, tissue factor is not expressed on
cellular surfaces in direct contact with circulating blood (see the
30
review listed in the References ). Presentation of tissue factor to the
circulation is the event that triggers the primary procoagulant pathway
of coagulation 31–33 (see Fig. 126.1). There are no known mutations
or deficiencies of human tissue factor, and tissue factor deletion in
34
mice is lethal during embryonic development, leading to the specu-
lation that tissue factor is essential for life.
Tissue factor activity is primarily regulated by controlling its
IIa presentation. The commonly accepted sources of functional tissue
Va factor are the subendothelium exposed upon vascular damage or
II TM C monocytes stimulated by cytokines. However, there is controversy
Xa regarding the source and presentation of active tissue factor and
whether functional tissue factor circulates in blood in healthy and
pathologic states. 35–40 Microparticle sources of blood-borne tissue
factor are generally defined as submicron-sized cell-derived membrane
fragments produced in response to activation or apoptosis. Their role
in hemostasis is still debated. 28,35,41,42
Prothrombinase Protein Case Thrombomodulin
Thrombomodulin is a type 1 transmembrane protein constitutively
Fig. 126.4 VITAMIN K–DEPENDENT COMPLEXES. Three procoagu- expressed on the surface of vascular endothelial cells (see Fig. 126.5A).
lant complexes (extrinsic tenase, intrinsic tenase, and prothrombinase) and Thrombomodulin is a high-affinity receptor for all thrombin forms
one anticoagulant complex (protein Case) are illustrated. Each membrane and acts as a cofactor for the thrombin-dependent activation of
complex consists of a vitamin K–dependent serine protease (factor VIIa protein C. The endothelial cell protein C receptor (EPCR) provides
43
[VIIa], factor IXa [IXa], factor Xa [Xa], or thrombin [IIa]) and a soluble or cell-specific binding sites for both protein C and activated protein C
cell surface–associated cofactor (factor VIIIa [heavy and light chain VIII H and (APC). 44–47 When bound to thrombomodulin, thrombin’s procoagu-
VIII L ], factor Va [heavy and light chain V H and V L ], tissue factor [TF], or lant activities (e.g., its capacity to generate fibrin and activate factor
thrombomodulin [TM]). Each serine protease is shown in association with V, factor VIII, factor XI, and platelets) are neutralized, and the rate
the appropriate cofactor protein and zymogen substrate(s) on the membrane of inactivation of thrombin by antithrombin is increased. 48–50 The
surface. The membrane serves as a scaffold for the coagulation reactants, generation of APC by the thrombin–thrombomodulin complex leads
4
9
enhancing the reaction rates by 10 –10 -fold.
to inactivation of the procoagulant cofactors factor Va and factor
VIIIa, thus suppressing thrombin formation. 51,52 Thrombin–
thrombomodulin, or the “protein Case” (see Fig. 126.4) complex,