Page 218 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 218
170 Part II Cellular Basis of Hematology
A B
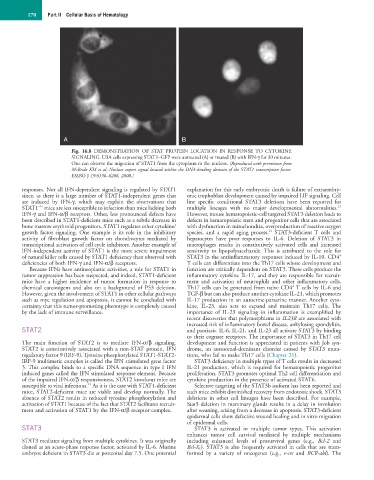
Fig. 16.8 DEMONSTRATION OF STAT PROTEIN LOCATION IN RESPONSE TO CYTOKINE
SIGNALING. U3A cells expressing STAT1–GFP were untreated (A) or treated (B) with IFN-γ for 30 minutes.
One can observe the migration of STAT1 from the cytoplasm to the nucleus. (Reproduced with permission from
McBride KM et al: Nuclear export signal located within the DNA-binding domain of the STAT1 transcription factor.
EMBO J 19:6196–6206, 2000.)
responses. Not all IFN-dependent signaling is regulated by STAT1 explanation for this early embryonic death is failure of extraembry-
since, as there is a large number of STAT1-independent genes that onic trophoblast development caused by impaired LIF signaling. Cell
are induced by IFN-γ, which may explain the observations that line specific conditional STAT3 deletions have been reported for
−/−
23
STAT1 mice are less susceptible to infection than mice lacking both multiple lineages with no major developmental abnormalities.
IFN-γ and IFN-α/β receptors. Other, less pronounced defects have However, mouse hematopoietic-cell targeted STAT3 deletion leads to
been described in STAT1-deficient mice such as a subtle decrease in defects in hematopoietic stem and progenitor cells that are associated
bone marrow erythroid progenitors. STAT1 regulates other cytokine/ with dysfunction in mitochondria, overproduction of reactive oxygen
24
growth factor signaling. One example is its role in the inhibitory species, and a rapid aging process. STAT3-deficient T cells and
activity of fibroblast growth factor on chondrocytes mediated by hepatocytes have poor responses to IL-6. Deletion of STAT3 in
transcriptional activation of cell cycle inhibitors. Another example of macrophages results in constitutively activated cells and increased
IFN-independent activity of STAT1 is the more severe impairment sensitivity to lipopolysaccharide. This is attributed to the role for
+
of natural killer cells caused by STAT1 deficiency than observed with STAT3 in the antiinflammatory responses induced by IL-10. CD4
deficiencies of both IFN-γ and IFN-α/β receptors. T cells can differentiate into the Th17 cells whose development and
Because IFNs have antineoplastic activities, a role for STAT1 in function are critically dependent on STAT3. These cells produce the
tumor suppression has been suspected, and indeed, STAT1-deficient inflammatory cytokine IL-17, and they are responsible for recruit-
mice have a higher incidence of tumor formation in response to ment and activation of neutrophils and other inflammatory cells.
+
chemical carcinogens and also on a background of P53 deletion. Th17 cells can be generated from naive CD4 T cells by IL-6 and
However, given the involvement of STAT1 in other cellular pathways TGF-β but can also produce another cytokine IL-21, which promotes
such as myc regulation and apoptosis, it cannot be concluded with IL-17 production in an autocrine-paracrine manner. Another cyto-
certainty that this tumor-promoting phenotype is completely caused kine, IL-23, also acts to expand and maintain Th17 cells. The
by the lack of immune surveillance. importance of IL-23 signaling in inflammation is exemplified by
recent discoveries that polymorphisms in IL23R are associated with
increased risk of inflammatory bowel disease, ankylosing spondylitis,
STAT2 and psoriasis. IL-6, IL-21, and IL-23 all activate STAT3 by binding
to their cognate receptors. The importance of STAT3 in Th17 cell
The main function of STAT2 is to mediate IFN-α/β signaling. development and function is appreciated in patients with Job syn-
STAT2 is constitutively associated with a non-STAT protein, IFN drome, an autosomal-dominant disorder caused by STAT3 muta-
regulatory factor 9 (IRF-9). Tyrosine phosphorylated STAT1-STAT2- tions, who fail to make Th17 cells (Chapter 21).
IRF-9 multimeric complex is called the IFN stimulated gene factor STAT3 deficiency in multiple types of T cells results in decreased
3. This complex binds to a specific DNA sequence in type I IFN IL-21 production, which is required for hematopoietic progenitor
induced genes called the IFN stimulated response element. Because proliferation. STAT3 promotes optimal Th2 cell differentiation and
of the impaired IFN-α/β responsiveness, STAT2 knockout mice are cytokine production in the presence of activated STAT6.
22
susceptible to viral infections. As it is the case with STAT1-deficient Selective targeting of the STAT3b isoform has been reported and
mice, STAT2-deficient mice are viable and develop normally. The such mice exhibit diminished recovery from endotoxic shock. STAT3
absence of STAT2 results in reduced tyrosine phosphorylation and deletions in other cell lineages have been described. For example,
activation of STAT1 because of the fact that STAT2 facilitates recruit- Stat3 deletion in mammary glands results in a delay in involution
ment and activation of STAT1 by the IFN-α/β receptor complex. after weaning, arising from a decrease in apoptosis. STAT3-deficient
epidermal cells show defective wound healing and in vitro migration
of epidermal cells.
STAT3 STAT3 is activated in multiple tumor types. This activation
enhances tumor cell survival mediated by multiple mechanisms
STAT3 mediates signaling from multiple cytokines. It was originally including enhanced levels of prosurvival genes (e.g., Bcl-2 and
cloned as an acute-phase response factor, activated by IL-6. Murine Bcl-X L ). STAT3 is also frequently activated in cells that are trans-
embryos deficient in STAT3 die at postcoital day 7.5. One potential formed by a variety of oncogenes (e.g., v-src and BCR-abl). The