Page 213 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 213
Chapter 16 Cytokine/Receptor Families and Signal Transduction 165
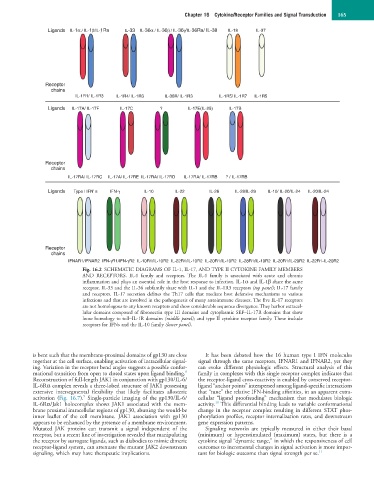
Ligands IL-1α / IL-1β/IL-1Ra IL-33 IL-36α / IL-36β / IL-36γ/IL-36Ra/ IL-38 IL-18 IL-37
Receptor
chains
IL-1R1/ IL-1R3 IL-1R4/ IL-1R3 IL-36R/ IL-1R3 IL-1R5/ IL-1R7 IL-1R5
Ligands IL-17A/ IL-17F IL-17C ? IL-17E(IL-25) IL-17B
Receptor
chains
IL-17RA/ IL-17RC IL-17A/ IL-17RE IL-17RA/ IL-17RD IL-17RA/ IL-17RB ? / IL-17RB
Ligands Type I IFN’ s IFN-γ IL-10 IL-22 IL-26 IL-28/IL-29 IL-10/ IL-20/IL-24 IL-20/IL-24
Receptor
chains
IFNAR1/IFNAR2 IFN-γR1/IFN-γR2 IL-10R1/IL-10R2 IL-22R1/IL-10R2 IL-20R1/IL-10R2 IL-28R1/IL-10R2 IL-20R1/IL-20R2 IL-22R1-IL-20R2
Fig. 16.2 SCHEMATIC DIAGRAMS OF IL-1, IL-17, AND TYPE II CYTOKINE FAMILY MEMBERS
AND RECEPTORS. IL-1 family and receptors. The IL-1 family is associated with acute and chronic
inflammation and plays an essential role in the host response to infection. IL-1α and IL-1β share the same
receptor. IL-33 and the IL-36 subfamily share with IL-1 and the IL-1R3 receptors (top panel); IL-17 family
and receptors. IL-17 secretion defines the Th17 cells that mediate host defensive mechanisms to various
infections and that are involved in the pathogenesis of many autoimmune diseases. The five IL-17 receptors
are not homologous to any known receptors and show considerable sequence divergence. They harbor extracel-
lular domains composed of fibronectin type III domains and cytoplasmic SEF–IL-17R domains that show
loose homology to toll–IL-1R domains (middle panel); and type II cytokine receptor family. These include
receptors for IFNs and the IL-10 family (lower panel).
is bent such that the membrane-proximal domains of gp130 are close It has been debated how the 16 human type I IFN molecules
together at the cell surface, enabling activation of intracellular signal- signal through the same receptors, IFNAR1 and IFNAR2, yet they
ing. Variation in the receptor bend angles suggests a possible confor- can evoke different physiologic effects. Structural analysis of this
8
mational transition from open to closed states upon ligand binding. family in complexes with this single receptor complex indicates that
Reconstruction of full-length JAK1 in conjunction with gp130/IL-6/ the receptor-ligand cross-reactivity is enabled by conserved receptor-
IL-6Rα complex reveals a three-lobed structure of JAK1 possessing ligand “anchor points” interspersed among ligand-specific interactions
extensive intersegmental flexibility that likely facilitates allosteric that “tune” the relative IFN-binding affinities, in an apparent extra-
9
activation (Fig. 16.7). Single-particle imaging of the gp130/IL-6/ cellular “ligand proofreading” mechanism that modulates biologic
10
IL-6Rα/Jak1 holocomplex shows JAK1 associated with the mem- activity. This differential binding leads to variable conformational
brane proximal intracellular regions of gp130, abutting the would-be change in the receptor complex resulting in different STAT phos-
inner leaflet of the cell membrane. JAK1 association with gp130 phorylation profiles, receptor internalization rates, and downstream
appears to be enhanced by the presence of a membrane environment. gene expression patterns.
Mutated JAK proteins can transmit a signal independent of the Signaling networks are typically measured in either their basal
receptor, but a recent line of investigation revealed that manipulating (minimum) or hyperstimulated (maximum) states, but there is a
the receptor by surrogate ligands, such as diabodies to mimic dimeric cytokine signal “dynamic range,” in which the responsiveness of cell
receptor-ligand system, can attenuate the mutant JAK2 downstream outcomes to incremental changes in signal activation is more impor-
signaling, which may have therapeutic implications. tant for biologic outcome than signal strength per se. 11