Page 217 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 217
Chapter 16 Cytokine/Receptor Families and Signal Transduction 169
Kinase
p-kinase
“Open”
SH2
FERM
SH2
10 nm
SH2
p-kinase
Kinase
SH2
“Closed”
FERM SH2
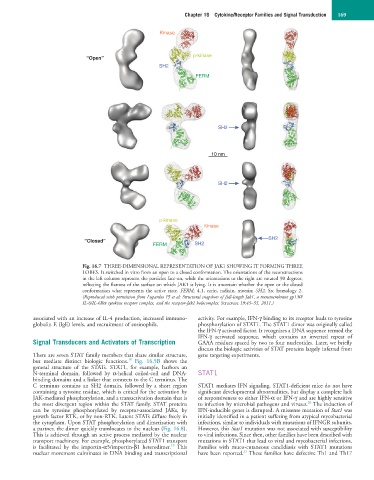
Fig. 16.7 THREE-DIMENSIONAL REPRESENTATION OF JAK1 SHOWING IT FORMING THREE
LOBES. It switched in vitro from an open to a closed conformation. The orientations of the reconstructions
in the left column represent the particles face-on, while the orientations to the right are rotated 90 degrees,
reflecting the flatness of the surface on which JAK1 is lying. It is uncertain whether the open or the closed
conformation what represents the active state. FERM, 4.1, ezrin, radixin, moesin; SH2, Src homology 2.
(Reproduced with permission from Lupardus PJ et al: Structural snapshots of full-length Jak1, a transmembrane gp130/
IL-6/IL-6Rα cytokine receptor complex, and the receptor-Jak1 holocomplex. Structure 19:45–55, 2011.)
associated with an increase of IL-4 production, increased immuno- activity. For example, IFN-γ binding to its receptor leads to tyrosine
globulin E (IgE) levels, and recruitment of eosinophils. phosphorylation of STAT1. The STAT1 dimer was originally called
the IFN-γ activated factor. It recognizes a DNA sequence termed the
IFN-γ activated sequence, which contains an inverted repeat of
Signal Transducers and Activators of Transcription GAAA residues spaced by two to four nucleotides. Later, we briefly
discuss the biologic activities of STAT proteins largely inferred from
There are seven STAT family members that share similar structure, gene targeting experiments.
18
but mediate distinct biologic functions. Fig. 16.5B shows the
general structure of the STATs. STAT1, for example, harbors an
N-terminal domain, followed by α-helical coiled-coil and DNA- STAT1
binding domains and a linker that connects to the C terminus. The
C terminus contains an SH2 domain, followed by a short region STAT1 mediates IFN signaling. STAT1-deficient mice do not have
containing a tyrosine residue, which is critical for the activation by significant developmental abnormalities, but display a complete lack
JAK-mediated phosphorylation, and a transactivation domain that is of responsiveness to either IFN-α or IFN-γ and are highly sensitive
20
the most divergent region within the STAT family. STAT proteins to infection by microbial pathogens and viruses. The induction of
can be tyrosine phosphorylated by receptor-associated JAKs, by IFN-inducible genes is disrupted. A missense mutation of Stat1 was
growth factor RTK, or by non-RTK. Latent STATs diffuse freely in initially identified in a patient suffering from atypical mycobacterial
the cytoplasm. Upon STAT phosphorylation and dimerization with infections, similar to individuals with mutations of IFNGR subunits.
a partner, the dimer quickly translocates to the nucleus (Fig. 16.8). However, this Stat1 mutation was not associated with susceptibility
This is achieved through an active process mediated by the nuclear to viral infections. Since then, other families have been described with
transport machinery. For example, phosphorylated STAT1 transport mutations in STAT1 that lead to viral and mycobacterial infections.
19
is facilitated by the importin-α5/importin-β1 heterodimer. This Families with muco-cutaneous candidiasis with STAT1 mutations
21
nuclear movement culminates in DNA binding and transcriptional have been reported. These families have defective Th1 and Th17