Page 2385 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2385
Chapter 144 Atherothrombosis 2127
An intriguing recent development in understanding the cellular for monocytes. Animal studies suggest that platelets may facilitate
composition of plaque comes from so-called lineage marker studies monocyte recruitment, acting as a bridge between the endothelium
21
that show that a surprisingly large percentage of foam cells expressing and circulating monocytes (see Fig. 144.5). Sophisticated single-cell
macrophage markers were actually derived from a smooth muscle cell imaging studies in mice showed that monocytes continue to traffic
20
lineage. These studies suggest that local cues in plaque might through the “shoulders” of even advanced stage lesions.
interact with intimal or adventitial smooth muscle cells to redirect Plaque generally grows in an eccentric pattern within the intima
their genetic programs towards a macrophage-like phenotype. It is and in certain instances can create significant obstruction to blood
possible that such cells do not possess the full migratory capacity of flow (Fig. 144.6, left). In such cases, as oxygen demand increases,
macrophages and this could contribute to foam cell trapping in tissue ischemia results, leading to angina and/or lower extremity
plaque. claudication. Recent in vivo studies using advanced imaging tech-
niques, such as intravascular ultrasound, however, demonstrate that
LESION EVOLUTION: REMODELING AND THE in most cases the vessel wall remodels as plaque grows (see Fig. 144.6,
right) so that the arterial lumen is mostly preserved and the bulk of
VULNERABLE PLAQUE the atheromatous mass is ablumenal. These studies show that tradi-
tional two-dimensional angiographic techniques vastly underestimate
In humans, plaque develops and evolves very slowly over many plaque burden. Of note, such studies, along with careful histopatho-
decades, explaining in part why animal models in which advanced logic examinations, have led to the concept that the “quality” of the
lesions develop rapidly over weeks and months may not accurately plaque may be more important than its quantity in predicting car-
reflect the human condition. Accumulation of foam cells is only one diovascular outcomes. Some plaques, particularly those with thick
component of the atherogenic process. Early on, signals from mac- fibrous caps and cellular cores, seem to be stable (Fig. 144.7), whereas
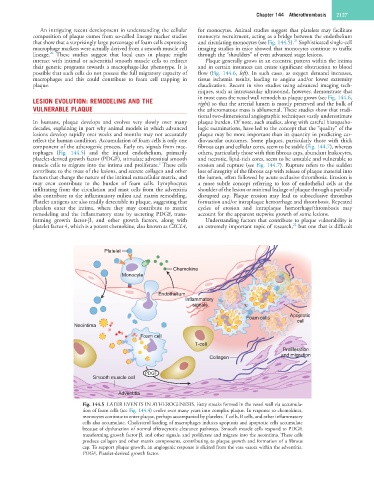
rophages (Fig. 144.5) and the injured endothelium, primarily others, particularly those with thin fibrous caps, abundant leukocytes,
platelet-derived growth factor (PDGF), stimulate adventitial smooth and necrotic, lipid-rich cores, seem to be unstable and vulnerable to
2
muscle cells to migrate into the intima and proliferate. These cells erosion and rupture (see Fig. 144.7). Rupture refers to the sudden
contribute to the mass of the lesions, and secrete collagen and other loss of integrity of the fibrous cap with release of plaque material into
factors that change the nature of the intimal extracellular matrix, and the lumen, often followed by acute occlusive thrombosis. Erosion is
may even contribute to the burden of foam cells. Lymphocytes a more subtle concept referring to loss of endothelial cells at the
infiltrating from the circulation and mast cells from the adventitia shoulder of the lesion or minimal leakage of plaque through a partially
also contribute to the inflammatory milieu and matrix remodeling. disrupted cap. Plaque erosion may lead to subocclusive thrombus
Platelet antigens are also readily detectable in plaque, suggesting that formation and/or intraplaque hemorrhage and thrombosis. Repeated
platelets enter the intima, where they may contribute to matrix cycles of erosion and intraplaque hemorrhage/thrombosis may
remodeling and the inflammatory state by secreting PDGF, trans- account for the apparent stepwise growth of some lesions.
forming growth factor-β, and other growth factors, along with Understanding factors that contribute to plaque vulnerability is
22
platelet factor 4, which is a potent chemokine, also known as CXCL4, an extremely important topic of research, but one that is difficult
Platelet
Chemokine
Monocyte
Endothelium
Inflammatory
signals
Apoptotic
Foam cells
cell
Neointima
Foam cell
T-cell
Proliferation
Collagen and migration
PDGF
Smooth muscle cell
Adventitia
Fig. 144.5 LATER EVENTS IN ATHEROGENESIS. Fatty streaks formed in the vessel wall via accumula-
tion of foam cells (see Fig. 144.4) evolve over many years into complex plaque. In response to chemokines,
monocytes continue to enter plaque, perhaps accompanied by platelets. T cells, B cells, and other inflammatory
cells also accumulate. Cholesterol loading of macrophages induces apoptosis and apoptotic cells accumulate
because of dysfunction of normal efferocytotic clearance pathways. Smooth muscle cells respond to PDGF,
transforming growth factor β, and other signals, and proliferate and migrate into the neointima. These cells
produce collagen and other matrix components, contributing to plaque growth and formation of a fibrous
cap. To support plaque growth, an angiogenic response is elicited from the vasa vasora within the adventitia.
PDGF, Platelet-derived growth factor.