Page 2387 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2387
Chapter 144 Atherothrombosis 2129
to model in animals. Some key features that have emerged are the
degree of angiogenesis within the plaque, balance of matrix-degrading
enzymes and enzyme inhibitors, level of apoptosis of cells within the Tissue factor
plaque, deposition of calcium within the plaque, and level of systemic Rupture
and local inflammation. OxLDL
Plaque angiogenesis is a recently appreciated process that can be
visualized by certain imaging modalities, such as ultrafast computed Platelet
tomography (CT) and magnetic resonance imaging (MRI), in real Tissue factor
+
23
time. Based on analogy to tumor growth, it is not surprising that Microparticle
the growing plaque requires a blood supply and also that the neoves-
sels within the plaque may, similar to their counterparts in cancer, be Fibrin clot
leaky and unstable. This neovascular instability may contribute to
plaque instability by facilitating entry of inflammatory cells, platelets,
and plasma components, such as fibrinogen and cell-derived mic-
roparticles (MPs). In animal models, treatment with antiangiogenic
agents significantly slows plaque growth. In some disease states, such
as diabetes, accelerated atherosclerosis may reflect a “microvascular”
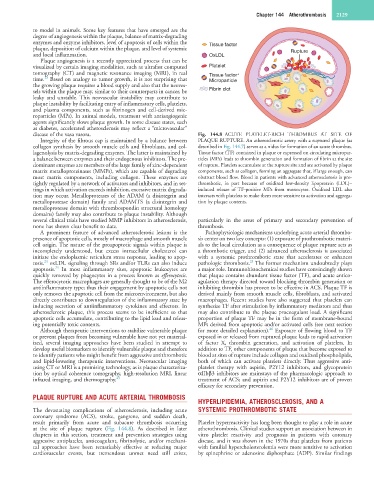
disease of the vasa vasora. Fig. 144.8 ACUTE PLATELET-RICH THROMBUS AT SITE OF
Integrity of the fibrous cap is maintained by a balance between PLAQUE RUPTURE. An atherosclerotic artery with a ruptured plaque (as
collagen synthesis by smooth muscle cells and fibroblasts, and col- described in Fig. 144.7) serves as a nidus for formation of an acute thrombus.
lagenolysis by matrix-degrading enzymes. The latter is maintained by Tissue factor (TF) contained in plaque or expressed on circulating micropar-
a balance between enzymes and their endogenous inhibitors. The pre- ticles (MPs) leads to thrombin generation and formation of fibrin at the site
dominant enzymes are members of the large family of zinc-dependent of rupture. Platelets accumulate at the rupture site and are activated by plaque
matrix metalloproteinases (MMPs), which are capable of degrading components, such as collagen, forming an aggregate that, if large enough, can
most matrix components, including collagen. These enzymes are obstruct blood flow. Blood in patients with advanced atherosclerosis is pro-
tightly regulated by a network of activators and inhibitors, and in set- thrombotic, in part because of oxidized low-density lipoprotein (LDL)–
tings in which activation exceeds inhibition, excessive matrix degrada- induced release of TF-positive MPs from monocytes. Oxidized LDL also
tion may occur. Metalloproteases of the ADAM (a disintegrin and interacts with platelets to make them more sensitive to activation and aggrega-
metalloprotease domain) family and ADAMTS (a disintegrin and tion by plaque contents.
metalloprotease domain with thrombospondin structural homology
domains) family may also contribute to plaque instability. Although
several clinical trials have studied MMP inhibitors in atherosclerosis, particularly in the areas of primary and secondary prevention of
none has shown clear benefit to date. thrombosis.
A prominent feature of advanced atherosclerotic lesions is the Pathophysiologic mechanisms underlying acute arterial thrombo-
presence of apoptotic cells, mostly of macrophage and smooth muscle sis center on two key concepts: (1) exposure of prothrombotic materi-
cell origin. The nature of the proapoptotic signals within plaque is als to the local circulation as a consequence of plaque rupture acts as
incompletely understood, but excess intracellular cholesterol can a thrombotic trigger, and (2) advanced atherosclerosis is associated
initiate the endoplasmic reticulum stress response, leading to apop- with a systemic prothrombotic state that accelerates or enhances
24
25
tosis. oxLDL signaling through SRs and/or TLRs can also induce pathologic thrombosis. The former mechanism undoubtedly plays
25
apoptosis. In most inflammatory sites, apoptotic leukocytes are a major role. Immunohistochemical studies have convincingly shown
quickly removed by phagocytes in a process known as efferocytosis. that plaque contains abundant tissue factor (TF), and acute antico-
The efferocytotic macrophages are generally thought to be of the M2 agulation therapy directed toward blocking thrombin generation or
antiinflammatory type; thus their engagement by apoptotic cells not inhibiting thrombin has proven to be effective in ACS. Plaque TF is
only removes the apoptotic cell from the microenvironment, but also derived mainly from smooth muscle cells, fibroblasts, and activated
directly contributes to downregulation of the inflammatory state by macrophages. Recent studies have also suggested that platelets can
inducing secretion of antiinflammatory cytokines and effectors. In synthesize TF after stimulation by inflammatory mediators and thus
atherosclerotic plaque, this process seems to be inefficient so that may also contribute to the plaque procoagulant load. A significant
apoptotic cells accumulate, contributing to the lipid load and releas- proportion of plaque TF may be in the form of membrane-bound
ing potentially toxic contents. MPs derived from apoptotic and/or activated cells (see next section
26
Although therapeutic interventions to stabilize vulnerable plaque for more detailed explanation). Exposure of flowing blood to TF
or prevent plaques from becoming vulnerable have not yet material- exposed in or released from ruptured plaque leads to rapid activation
ized, several imaging approaches have been studied in attempt to of factor X, thrombin generation, and activation of platelets. In
develop useful biomarkers to identify vulnerable plaque and therefore addition to TF, other components of plaque that become exposed to
to identify patients who might benefit from aggressive antithrombotic blood at sites of rupture include collagen and oxidized phospholipids,
and lipid-lowering therapeutic interventions. Neovascular imaging both of which can activate platelets directly. Thus aggressive anti-
using CT or MRI is a promising technology, as is plaque characteriza- platelet therapy with aspirin, P2Y12 inhibitors, and glycoprotein
tion by optical coherence tomography, high-resolution MRI, linear αIIbβ3 inhibitors are mainstays of the pharmacologic approach to
infrared imaging, and thermography. 23 treatment of ACS; and aspirin and P2Y12 inhibitors are of proven
efficacy for secondary prevention.
PLAQUE RUPTURE AND ACUTE ARTERIAL THROMBOSIS
HYPERLIPIDEMIA, ATHEROSCLEROSIS, AND A
The devastating complications of atherosclerosis, including acute SYSTEMIC PROTHROMBOTIC STATE
coronary syndrome (ACS), stroke, gangrene, and sudden death,
result primarily from acute and subacute thrombosis occurring Platelet hyperreactivity has long been thought to play a role in acute
at the site of plaque rupture (Fig. 144.8). As described in later atherothrombosis. Clinical studies support an association between in
chapters in this section, treatment and prevention strategies using vitro platelet reactivity and prognosis in patients with coronary
aggressive antiplatelet, anticoagulant, fibrinolytic, and/or mechani- disease, and it was shown in the 1970s that platelets from patients
cal approaches have been remarkably effective at reducing major with familial hypercholesterolemia were more sensitive to activation
cardiovascular events, but tremendous unmet need still exists, by epinephrine or adenosine diphosphate (ADP). Similar findings