Page 2382 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2382
2124 Part XII Hemostasis and Thrombosis
miR33a
Cholesterol Hepatocyte
Macrophage
Nascent HDL
Mature HDL
miR33a
Statins Peripheral cell
CETP +
TG
ABCA1 Cholesterol
Statins
ABCG1 SRB-1
Apo B
Apo A LDL receptor LDL/VLDL
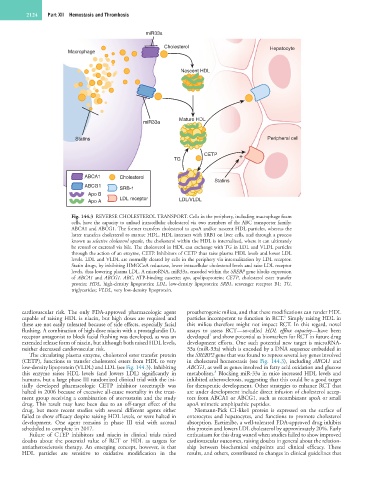
Fig. 144.3 REVERSE CHOLESTEROL TRANSPORT. Cells in the periphery, including macrophage foam
cells, have the capacity to unload intracellular cholesterol via two members of the ABC transporter family:
ABCA1 and ABCG1. The former transfers cholesterol to apoA and/or nascent HDL particles, whereas the
latter transfers cholesterol to mature HDL. HDL interacts with SRB1 on liver cells, and through a process
known as selective cholesterol uptake, the cholesterol within the HDL is internalized, where it can ultimately
be reused or excreted via bile. The cholesterol in HDL can exchange with TG in LDL and VLDL particles
through the action of an enzyme, CETP. Inhibitors of CETP thus raise plasma HDL levels and lower LDL
levels. LDL and VLDL are normally cleared by cells in the periphery via internalization by LDL receptor.
Statin drugs, by inhibiting HMGCoA reductase, lower intracellular cholesterol levels and raise LDL receptor
levels, thus lowering plasma LDL. A microRNA, miR33a, encoded within the SREBP gene blocks expression
of ABCA1 and ABCG1. ABC, ATP-binding cassette; apo, apolipoprotein; CETP, cholesterol ester transfer
protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SRB1, scavenger receptor B1; TG,
triglycerides; VLDL, very low-density lipoprotein.
cardiovascular risk. The only FDA-approved pharmacologic agent proatherogenic milieu, and that these modifications can render HDL
6
capable of raising HDL is niacin, but high doses are required and particles incompetent to function in RCT. Simply raising HDL in
these are not easily tolerated because of side effects, especially facial this milieu therefore might not impact RCT. In this regard, novel
flushing. A combination of high-dose niacin with a prostaglandin D 2 assays to assess RCT—so-called HDL efflux capacity—have been
7
receptor antagonist to block facial flushing was developed, as was an developed and show potential as biomarkers for RCT in future drug
extended release form of niacin, but although both raised HDL levels, development efforts. One such potential new target is microRNA-
neither decreased cardiovascular risk. 33a (miR-33a) which is encoded by a DNA sequence embedded in
The circulating plasma enzyme, cholesterol ester transfer protein the SREBP2 gene that was found to repress several key genes involved
(CETP), functions to transfer cholesterol esters from HDL to very in cholesterol homeostasis (see Fig. 144.3), including ABCA1 and
low-density lipoprotein (VLDL) and LDL (see Fig. 144.3). Inhibiting ABCG1, as well as genes involved in fatty acid oxidation and glucose
8
this enzyme raises HDL levels (and lowers LDL) significantly in metabolism. Blocking miR-33a in mice increased HDL levels and
humans, but a large phase III randomized clinical trial with the ini- inhibited atherosclerosis, suggesting that this could be a good target
tially developed pharmacologic CETP inhibitor torcetrapib was for therapeutic development. Other strategies to enhance RCT that
halted in 2006 because of excessive all-cause mortality in the treat- are under development include direct infusion of cholesterol accep-
ment group receiving a combination of atorvastatin and the study tors from ABCA1 or ABCG1, such as recombinant apoA or small
drug. This result may have been due to an off-target effect of the apoA mimetic amphipathic peptides.
drug, but more recent studies with several different agents either Niemann-Pick C1-like1 protein is expressed on the surface of
failed to show efficacy despite raising HDL levels, or were halted in enterocytes and hepatocytes, and functions to promote cholesterol
development. One agent remains in phase III trial with accrual absorption. Ezetimibe, a well-tolerated FDA-approved drug inhibits
scheduled to complete in 2017. this protein and lowers LDL cholesterol by approximately 20%. Early
Failure of CTEP inhibitors and niacin in clinical trials raised enthusiasm for this drug waned when studies failed to show improved
doubts about the potential value of RCT or HDL as targets for cardiovascular outcomes, raising doubts in general about the relation-
antiatherosclerosis therapy. An emerging concept, however, is that ship between biochemical endpoints and clinical efficacy. These
HDL particles are sensitive to oxidative modification in the results, and others, contributed to changes in clinical guidelines that