Page 321 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 321
Chapter 24 Complement and Immunoglobulin Biology Leading to Clinical Translation 263
Alternative Pathway MG8 domains, which is nevertheless close to the protein’s surface.
The subsequent determination of the atomic structure of the activated
The AP may represent one of the earliest forms of innate immunity. form of C3 (i.e., C3b) demonstrated a dramatic shift in the location
Unlike the CP or LP pathway, the AP can be fully activated in the of the TED. 24,25 Proteolytic cleavage releases the C3a anaphylatoxin
absence of specific pathogen binding by a “recognition” equivalent to peptide, and the TED becomes fully exposed to engage potential
21
C1q or MBL. In fact, the AP is always “on” at a low level. In targets (see structure-based depiction of C3b in Fig. 24.2B). Thus the
addition, the AP forms and uses the distinct C3 convertase C3bBb. 22 dramatic shift in structure also exposes potential binding sites for
Complement C3 is a two-chain protein with an apparent molecu- factor B of the AP and competing sites for regulators of C3b, such
lar weight of approximately 200 kDa. The crystal structure of native as factor H (FH), membrane cofactor protein (MCP), complement
C3, shown as a domain-colored ribbon model in Fig. 24.2A, identi- receptor type 1 (CR1), and decay accelerating factor (DAF; all
fied 13 distinct domains, including the thioester domain (TED), described later in this section). At a low so-called “tickover” level, the
23
which contained the covalent binding site. In the native molecule, thioester bond undergoes spontaneous hydrolysis, forming C3(H 2O).
the intramolecular thioester bond, formed between the side chains of This conformationally altered C3b-like form of C3 (see Fig. 24.2B)
cysteine and glutamine residues within the sequence CGEQ, is allows for binding to factor B, a plasma protein. Factor B is a serine
buried within a hydrophobic interface formed between the TED and protease that is approximately 30% identical to C2. The binding of
C3(H 2 O)* C3(H 2 O)
C3
C345C
“Tick-over mechanism”
CUB Anchor C3 C3c
MG8 iC3b
MG7
α’NT
ANA C3f
TED
Thioester MG3
C3a
MG2 C3b iC3b C3dg C3d
MG6
MG4
MG1
A LNK MG5 B
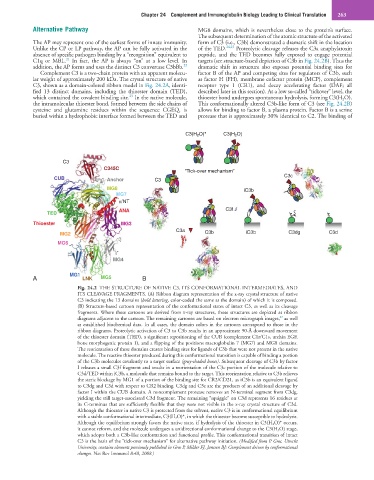
Fig. 24.2 THE STRUCTURE OF NATIVE C3, ITS CONFORMATIONAL INTERMEDIATES, AND
ITS CLEAVAGE FRAGMENTS. (A) Ribbon diagram representation of the x-ray crystal structure of native
C3 indicating the 13 domains (bold lettering, color-coded the same as the domain) of which it is composed.
(B) Structure-based cartoon representation of the conformational states of intact C3, as well as its cleavage
fragments. Where these cartoons are derived from x-ray structures, those structures are depicted as ribbon
27
diagrams adjacent to the cartoon. The remaining cartoons are based on electron micrograph images, as well
as established biochemical data. In all cases, the domain colors in the cartoons correspond to those in the
ribbon diagrams. Proteolytic activation of C3 to C3b results in an approximate 90-Å downward movement
of the thioester domain (TED), a significant repositioning of the CUB (complement C1r/C1s, urchin EGF,
bone morphogenic protein 1), and a flipping of the positions macroglobulin 7 (MG7) and MG8 domains.
The reorientation of these domains creates binding sites for ligands of C3b that were not present in the native
molecule. The reactive thioester produced during this conformational transition is capable of binding a portion
of the C3b molecules covalently to a target surface (gray-shaded boxes). Subsequent cleavage of C3b by factor
I releases a small C3f fragment and results in a reorientation of the C3c portion of the molecule relative to
C3d/TED within iC3b, a molecule that remains bound to the target. This reorientation relative to C3b relieves
the steric blockage by MG1 of a portion of the binding site for CR2/CD21, as iC3b is an equivalent ligand
to C3dg and C3d with respect to CR2 binding. C3dg and C3c are the products of an additional cleavage by
factor I within the CUB domain. A noncomplement protease removes an N-terminal segment from C3dg,
yielding the still target-associated C3d fragment. The remaining “squiggle” on C3d represents 16 residues at
its C-terminus that are sufficiently flexible that they were not visible in the x-ray crystal structure of C3d.
Although the thioester in native C3 is protected from the solvent, native C3 is in conformational equilibrium
with a stable conformational intermediate, C3(H 2 O)*, in which the thioester become susceptible to hydrolysis.
Although the equilibrium strongly favors the native state, if hydrolysis of the thioester in C3(H 2 O)* occurs,
it cannot reform, and the molecule undergoes a unidirectional conformational change to the C3(H 2 O) stage,
which adopts both a C3b-like conformation and functional profile. This conformational transition of intact
C3 is the basis of the “tick-over mechanism” for alternative pathway initiation. (Modified from P. Gros, Utrecht
University; contains elements previously published in Gros P, Milder FJ, Janssen BJ: Complement driven by conformational
changes. Nat Rev Immunol 8:48, 2008.)