Page 401 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 401
Chapter 27 Granulocytopoiesis and Monocytopoiesis 323
CONTROL OF GRANULOPOIESIS
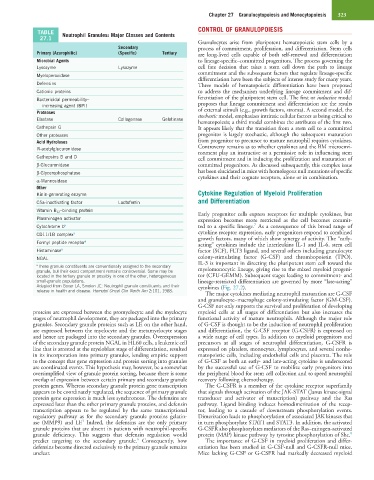
TABLE Neutrophil Granules: Major Classes and Contents
27.1
Granulocytes arise from pluripotent hematopoietic stem cells by a
Secondary process of commitment, proliferation, and differentiation. Stem cells
Primary (Azurophilic) (Specific) Tertiary are long-lived cells capable of both self-renewal and differentiation
Microbial Agents to lineage-specific–committed progenitors. The process governing the
Lysozyme Lysozyme cell fate decision that takes a stem cell down the path to lineage
commitment and the subsequent factors that regulate lineage-specific
Myeloperoxidase
differentiation have been the subjects of intense study for many years.
Defensins Three models of hematopoietic differentiation have been proposed
Cationic proteins to address the mechanism underlying lineage commitment and dif-
Bactericidal permeability– ferentiation of the pluripotent stem cell. The first or inductive model
increasing agent (BPI) proposes that lineage commitment and differentiation are the results
Proteases of external stimuli (e.g., growth factors, stroma). A second model, the
stochastic model, emphasizes intrinsic cellular factors as being critical to
Elastase Collagenase Gelatinase
hematopoiesis; a third model combines the attributes of the first two.
Cathepsin G It appears likely that the transition from a stem cell to a committed
Other proteases progenitor is largely stochastic, although the subsequent maturation
Acid Hydrolases from progenitor to precursor to mature neutrophil requires cytokines.
N-acetylglucuronidase Controversy remains as to whether cytokines and the BM microenvi-
ronment play an instructive or a permissive role in influencing stem
Cathepsins B and D cell commitment and in inducing the proliferation and maturation of
β-Glucuronidase committed progenitors. As discussed subsequently, this complex issue
β-Glycerophosphatase has been elucidated in mice with homologous null mutations of specific
α-Mannosidase cytokines and their cognate receptors, alone or in combination.
Other
Kinin-generating enzyme Cytokine Regulation of Myeloid Proliferation
C5a-inactivating factor Lactoferrin and Differentiation
Vitamin B 12 –binding protein
Early progenitor cells express receptors for multiple cytokines, but
Plasminogen activator expression becomes more restricted as the cell becomes commit-
7
Cytochrome b a ted to a specific lineage. As a consequence of this broad range of
CD11/1B complex a cytokine receptor expression, early progenitors respond to combined
growth factors, many of which show synergy of activity. The “early-
Formyl peptide receptor a acting” cytokines include the interleukins IL-1 and IL-6, stem cell
Histaminase a factor (SCF), FLT3 ligand, and several others including granulocyte
NGAL colony-stimulating factor (G-CSF) and thrombopoietin (TPO).
IL-3 is important in directing the pluripotent stem cell toward the
a These granule constituents are conventionally assigned to the secondary
granule, but their exact compartment remains controversial. Some may be myelomonocytic lineage, giving rise to the mixed myeloid progeni-
located in the tertiary granule or possibly in one of the other, heterogeneous tor (CFU-GEMM). Subsequent stages leading to commitment- and
small-granule populations. lineage-restricted differentiation are governed by more “late-acting”
Adapted from Boxer LA, Smolen JE: Neutrophil granule constituents and their cytokines (Fig. 27.2).
release in health and disease. Hematol Oncol Clin North Am 2:101, 1988.
The major cytokines mediating neutrophil maturation are G-CSF
and granulocyte–macrophage colony-stimulating factor (GM-CSF).
G-CSF not only supports the survival and proliferation of developing
proteins are expressed between the promyelocyte and the myelocyte myeloid cells at all stages of differentiation but also increases the
stages of neutrophil development, they are packaged into the primary functional activity of mature neutrophils. Although the major role
granules. Secondary granule proteins such as LF, on the other hand, of G-CSF is thought to be the induction of neutrophil proliferation
are expressed between the myelocyte and the metamyelocyte stages and differentiation, the G-CSF receptor (G-CSFR) is expressed on
and hence are packaged into the secondary granules. Overexpression a wide range of cell types. In addition to myeloid progenitors and
of the secondary granule protein NGAL in HL60 cells, a leukemic cell precursors at all stages of neutrophil differentiation, G-CSFR is
line that is arrested at the myeloblast stage of differentiation, resulted expressed on platelets, monocytes, lymphocytes, and several nonhe-
in its incorporation into primary granules, lending empiric support matopoietic cells, including endothelial cells and placenta. The role
to the concept that gene expression and protein sorting into granules of G-CSF as both an early- and late-acting cytokine is underscored
are coordinated events. This hypothesis may, however, be a somewhat by the successful use of G-CSF to mobilize early progenitors into
oversimplified view of granule protein sorting, because there is some the peripheral blood for stem cell collection and to speed neutrophil
overlap of expression between certain primary and secondary granule recovery following chemotherapy.
protein genes. Whereas secondary granule protein gene transcription The G-CSFR is a member of the cytokine receptor superfamily
appears to be coordinately regulated, the sequence of primary granule that signals through activation of the JAK-STAT (Janus kinase-signal
protein gene expression is much less synchronous. The defensins are transducer and activator of transcription) pathway and the Ras
expressed later than the other primary granule proteins, and defensin pathway. Ligand binding induces homodimerization of the recep-
transcription appears to be regulated by the same transcriptional tor, leading to a cascade of downstream phosphorylation events.
regulatory pathway as for the secondary granule proteins gelatin- Dimerization leads to phosphorylation of associated JAK kinases that
5
ase (MMP9) and LF. Indeed, the defensins are the only primary in turn phosphorylate STAT1 and STAT3. In addition, the activated
granule proteins that are absent in patients with neutrophil-specific G-CSFR also phosphorylates mediators of the Ras–mitogen-activated
granule deficiency. This suggests that defensin regulation would protein (MAP) kinase pathway by tyrosine phosphorylation of Shc. 8
6
predict targeting to the secondary granule. Consequently, how The importance of G-CSF in myeloid proliferation and differ-
defensins become directed exclusively to the primary granule remains entiation has been studied in G-CSF-null and G-CSFR-null mice.
unclear. Mice lacking G-CSF or G-CSFR had markedly decreased myeloid