Page 456 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 456
Chapter 29 Inherited Bone Marrow Failure Syndromes 377
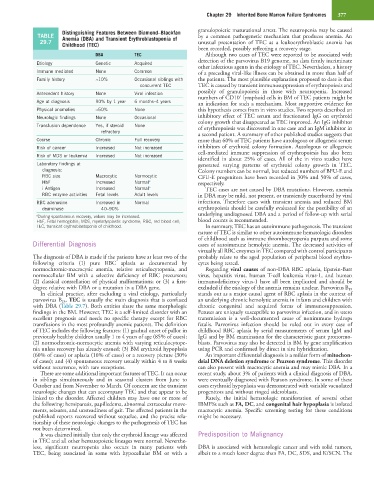
Distinguishing Features Between Diamond–Blackfan granulopoietic maturational arrest. The neutropenia may be caused
TABLE Anemia (DBA) and Transient Erythroblastopenia of by a common pathogenetic mechanism that produces anemia. An
29.7 Childhood (TEC) unusual presentation of TEC as a leukoerythroblastic anemia has
been recorded, possibly reflecting a recovery stage.
DBA TEC Although two cases of TEC were reported to be associated with
detection of the parvovirus B19 genome, no data firmly incriminate
Etiology Genetic Acquired
other infectious agents in the etiology of TEC. Nevertheless, a history
Immune mediated None Common of a preceding viral-like illness can be obtained in more than half of
Family history ≈10% Occasional siblings with the patients. The most plausible explanation proposed to date is that
concurrent TEC TEC is caused by transient immunosuppression of erythropoiesis and
Antecedent history None Viral infection possibly of granulopoiesis in those with neutropenia. Increased
+
numbers of CD10 lymphoid cells in BM of TEC patients might be
Age at diagnosis 90% by 1 year 6 months–4 years an indication for such a mechanism. Most supportive evidence for
Physical anomalies ≈50% None this hypothesis comes from in vitro studies. Two reports described an
Neurologic findings None Occasional inhibitory effect of TEC serum and fractionated IgG on erythroid
colony growth that disappeared as TEC improved. An IgG inhibitor
Transfusion dependence Yes, if steroid None of erythropoiesis was discovered in one case and an IgM inhibitor in
refractory
a second patient. A summary of other published studies suggests that
Course Chronic Full recovery more than 60% of TEC patients have autologous or allogeneic serum
Risk of cancer Increased Not increased inhibitors of erythroid colony formation. Autologous or allogeneic
Risk of MDS or leukemia Increased Not increased cell-mediated immune suppression of erythropoiesis has also been
identified in about 25% of cases. All of the in vitro studies have
Laboratory findings at generated varying patterns of erythroid colony growth in TEC.
diagnosis: Colony numbers can be normal, but reduced numbers of BFU-E and
RBC size Macrocytic Normocytic CFU-E progenitors have been recorded in 30% and 50% of cases,
HbF Increased Normal a respectively.
i Antigen Increased Normal a TEC cases are not caused by DBA mutations. However, anemia
RBC enzyme activities Fetal levels Adult levels in DBA may be mild, not present, or transiently exacerbated by viral
RBC adenosine Increased in Normal infections. Therefore cases with transient anemia and reduced BM
deaminase 40–90% erythropoiesis should be carefully evaluated for the possibility of an
underlying undiagnosed DBA and a period of follow-up with serial
a During spontaneous recovery, values may be increased.
HbF, Fetal hemoglobin; MDS, myelodysplastic syndrome; RBC, red blood cell; blood counts is recommended.
TEC, transient erythroblastopenia of childhood. In summary, TEC has an autoimmune pathogenesis. The transient
nature of TEC is similar to other autoimmune hematologic disorders
of childhood such as immune thrombocytopenia purpura and some
Differential Diagnosis cases of autoimmune hemolytic anemia. The decreased activities of
virtually all RBC enzymes in TEC compared with control participants
The diagnosis of DBA is made if the patients have at least two of the probably relate to the aged population of peripheral blood erythro-
following criteria (1) pure RBC aplasia as documented by cytes being tested.
normochromic-macrocytic anemia, relative reticulocytopenia, and Regarding viral causes of non-DBA RBC aplasia, Epstein-Barr
normocellular BM with a selective deficiency of RBC precursors; virus, hepatitis virus, human T-cell leukemia virus-1, and human
(2) classical constellation of physical malformations; or (3) a first- immunodeficiency virus-1 have all been implicated and should be
degree relative with DBA or a mutation in a DBA gene. excluded if the etiology of the anemia remains unclear. Parvovirus B 19
In clinical practice, after excluding a viral etiology, particularly stands out as a major causal agent of RBC aplasia in the context of
parvovirus B 19 , TEC is usually the main diagnosis that is confused an underlying chronic hemolytic anemia in infants and children with
with DBA (Table 29.7). Both entities share the same morphologic chronic congenital and acquired forms of immunosuppression.
findings in the BM. However, TEC is a self-limited disorder with an Fetuses are uniquely susceptible to parvovirus infection, and in utero
excellent prognosis and needs no specific therapy except for RBC transmission is a well-documented cause of nonimmune hydrops
transfusions in the most profoundly anemic patients. The definition fetalis. Parvovirus infection should be ruled out in every case of
of TEC includes the following features: (1) gradual onset of pallor in childhood RBC aplasia by serial measurements of serum IgM and
previously healthy children usually 1 to 4 years of age (85% of cases); IgG and by BM examination for the characteristic giant pronormo-
(2) normochromic-normocytic anemia with varying reticulocytope- blasts. Parvovirus may also be detected in BM by gene amplification
nia unless recovery has already ensued; (3) BM erythroid hypoplasia using PCR and confirmed by direct in situ hybridization.
(60% of cases) or aplasia (10% of cases) or a recovery picture (30% An important differential diagnosis is a milder form of mitochon-
of cases); and (4) spontaneous recovery usually within 4 to 8 weeks drial DNA deletion syndrome or Pearson syndrome. This disorder
without recurrence, with rare exceptions. can also present with macrocytic anemia and may mimic DBA. In a
There are some additional important features of TEC. It can occur recent study, about 3% of patients with a clinical diagnosis of DBA,
in siblings simultaneously and in seasonal clusters from June to were eventually diagnosed with Pearson syndrome. In some of these
October and from November to March. Of concern are the transient cases erythroid hypoplasia was demonstrated with variable vacuolated
neurologic changes that can accompany TEC and that appear to be progenitors and without ringed sideroblasts.
linked to the disorder. Affected children may have one or more of Rarely, the initial hematologic manifestation of several other
the following: hemiparesis, papilledema, abnormal extraocular move- IBMFSs such as FA, DC, and congenital hair hypoplasia is isolated
ments, seizures, and unsteadiness of gait. The affected patients in the macrocytic anemia. Specific screening testing for these conditions
published reports recovered without sequelae, and the precise rela- might be necessary.
tionship of these neurologic changes to the pathogenesis of TEC has
not been determined.
It was claimed initially that only the erythroid lineage was affected Predisposition to Malignancy
in TEC and all other hematopoietic lineages were normal. Neverthe-
less, significant neutropenia also occurs in many patients with DBA is associated with hematologic cancer and with solid tumors,
TEC, being associated in some with hypocellular BM or with a albeit to a much lesser degree than FA, DC, SDS, and K/SCN. The