Page 476 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 476
Chapter 30 Aplastic Anemia 397
autosomal dominant patients with this constitutional BM failure function is implicit in the success of BM transplantation in AA
syndrome provided a genetic basis for DKC. Central to the repair because important stromal elements remain of host origin.
machinery is an RNA template, encoded by TERC, on which telom-
erase, a reverse transcriptase encoded by TERT, elongates the nucleo-
tide repeat structure; other proteins, including the DKC1 gene PATHOPHYSIOLOGIC PATHWAYS LEADING TO
product dyskenin, are associated with the telomere repair complex. APLASTIC ANEMIA
Systematic surveys of DNA disclosed first TERC and later TERT
mutations in some patients with apparently acquired AA, including Direct Hematopoietic Injury
4
older adults. Family members who share the mutation, despite
normal or near-normal blood counts, have hypocellular marrows, The most common form of AA is iatrogenic; transient BM failure
+
reduced CD34 cell counts and poor hematopoietic colony forma- routinely follows treatment with cytotoxic chemotherapeutic drugs
tion, increased hematopoietic growth factor levels, and of course or irradiation (Fig. 30.5). Certain chemical or physical agents directly
short telomeres. However, clinical presentation is much later than in injure proliferating and quiescent hematopoietic cells. However,
typical DKC, and physical anomalies are often absent. Chromosomes patients with community-acquired AA rarely have a history of expo-
are also protected by several proteins that bind directly to telomeres. sure to such physicochemical agents. Even benzene, which can act as
Mutations in the gene for shelterin, one such protein, produce very a particularly inefficient cytotoxic chemical, is an infrequent cause of
severe DKC. Some inherited sequence variants/polymorphism in AA in developed countries. Medical drugs are associated with acquired
genes that repair or protect telomeres appear to be genetic risk factors AA, and in some instances, they can directly cause BM damage.
in acquired AA, probably because they confer a quantitatively reduced However, compared with chemotherapeutic agents, which are deliv-
hematopoietic stem cell compartment that may also be qualitatively ered in high doses, relatively low total quantities of ingested drug
inadequate to sustain immune-mediated damage. Accelerated telo- apparently cause idiosyncratic hematologic reactions. In addition to
mere attrition in AA not currently explained by mutations may be their direct toxic effects, chemicals and viruses may induce complex
caused by subtle or obscure genetic lesions or follow from the patho- and not well-understood immune reactions leading to BM failure in
physiology of BM stress and excessive stem cell turnover. persons with AA (see Fig. 30.5).
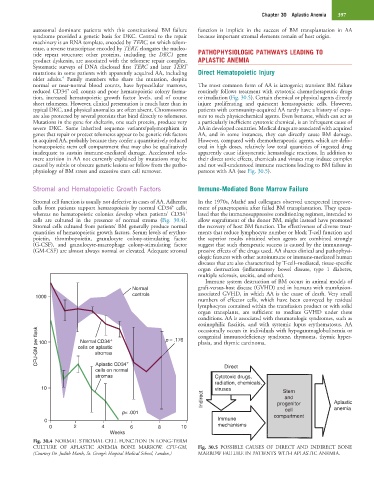
Stromal and Hematopoietic Growth Factors Immune-Mediated Bone Marrow Failure
Stromal cell function is usually not defective in cases of AA. Adherent In the 1970s, Mathé and colleagues observed unexpected improve-
+
cells from patients support hematopoiesis by normal CD34 cells, ment of pancytopenia after failed BM transplantation. They specu-
+
whereas no hematopoietic colonies develop when patients’ CD34 lated that the immunosuppressive conditioning regimen, intended to
cells are cultured in the presence of normal stroma (Fig. 30.4). allow engraftment of the donor BM, might instead have promoted
Stromal cells cultured from patients’ BM generally produce normal the recovery of host BM function. The effectiveness of diverse treat-
quantities of hematopoietic growth factors. Serum levels of erythro- ments that reduce lymphocyte number or block T-cell function and
poietin, thrombopoietin, granulocyte colony-stimulating factor the superior results obtained when agents are combined strongly
(G-CSF), and granulocyte-macrophage colony-stimulating factor suggest that such therapeutic success is caused by the immunosup-
(GM-CSF) are almost always normal or elevated. Adequate stromal pressive effects of the drugs used. AA shares clinical and pathophysi-
ologic features with other autoimmune or immune-mediated human
diseases that are also characterized by T-cell–mediated, tissue-specific
organ destruction (inflammatory bowel disease, type 1 diabetes,
multiple sclerosis, uveitis, and others).
Immune system destruction of BM occurs in animal models of
Normal graft-versus-host disease (GVHD) and in humans with transfusion-
1000 controls associated GVHD, in which AA is the cause of death. Very small
numbers of effector cells, which have been conveyed by residual
lymphocytes contained within the transfusion product or with solid
organ transplants, are sufficient to mediate GVHD under these
conditions. AA is associated with rheumatologic syndromes, such as
eosinophilic fasciitis, and with systemic lupus erythematosus. AA
CFU-GM per flask 100 cells on aplastic + p = .176 congenital immunodeficiency syndrome, thymoma, thymic hyper-
occasionally occurs in individuals with hypogammaglobulinemia or
Normal CD34
plasia, and thymic carcinoma.
stromas
Aplastic CD34
cells on normal + Direct
stromas Cytotoxic drugs,
radiation, chemicals,
10 viruses Stem
Indirect progenitor Aplastic
and
cell
p< .001 compartment anemia
0 Immune
0 2 4 6 8 10 mechanisms
Weeks
Fig. 30.4 NORMAL STROMAL CELL FUNCTION IN LONG-TERM
CULTURE OF APLASTIC ANEMIA BONE MARROW. CFU-GM, Fig. 30.5 POSSIBLE CAUSES OF DIRECT AND INDIRECT BONE
(Courtesy Dr. Judith Marsh, St. George’s Hospital Medical School, London.) MARROW FAILURE IN PATIENTS WITH APLASTIC ANEMIA.