Page 483 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 483
404 Part IV Disorders of Hematopoietic Cell Development
diagnosis. Patients with severe liver disease and splenomegaly, sys- specimen should be at least 1 cm long. There should no hesitation
temic lupus erythematosus, or overwhelming sepsis can have low in performing a second procedure if required.
blood cell counts, but the clinical presentation is not subtle. Similarly, BM cellularity is best estimated from the core biopsy. Point count-
BM aplasia following cytotoxic drug therapy for cancers and a variety ing under microscopic cross hairs in many parts of a histologic section
of nonmalignant diseases is anticipated. In the challenging case, is the most accurate method of determining cellularity, but hematolo-
obvious medical causes of pancytopenia have usually already been gists commonly rely on visual estimation only. A crude “eyeball”
excluded. Pancytopenia almost never results from peripheral blood approximation is almost always adequate in severe aplasia, because
cell destruction alone. In AA, the blood smear does not show reticu- the hematopoietic content of the BM specimen is usually close to
locytes, band forms, or the large platelets typical of increased com- zero. Estimates of BM cellularity based on examination of the aspirate
pensatory BM efforts. smear and biopsy specimen are correlated, but dilution of the aspirate
Acquired AA is a disease of the young, as is constitutional aplasia. by sinusoidal blood often occurs, and the aspirate can be hypocellular
Patients with FA often, but not always, have physical abnormalities. when the biopsy specimen is hypercellular or can show focal areas of
In the absence of a suggestive family history or the presence of active hematopoiesis. Normal BM cellularity decreases considerably
physical anomalies, the distinction between acquired and constitu- with age, a variation that is of some importance in assessing the older
tional disease depends on the results of a clastogenic-stress culture of patient with aplasia or myelodysplasia. In autopsy samples from
peripheral lymphocytes (for FA) and telomere length of leukocytes normal, young children, approximately 80% of the BM space of the
(for DKC and the telomeropathies). iliac crest is cellular. BM cellularity gradually decreases from age 20
In older patients the major differential diagnosis is between AA to 70 years and more precipitously in the very elderly, to approxi-
and myelodysplasia. There is a gray area between hypocellular myelo- mately 30% in the eighth decade of life. For practical purposes, the
dysplasia and moderate AA, and even competent hematologists might lower limit of normal BM cellularity in adults is accepted at approxi-
not agree on the final diagnosis. BM cytogenetics can help in estab- mately 30%, but the differences at the extremes of life should be
lishing the proper diagnosis. recalled when evaluating infants and the elderly. In most patients with
Myelofibrosis can also produce pancytopenia, but the BM is not AA, total BM cellularity is extremely low, but there can be significant
aspirable, the spleen is often enlarged, and the peripheral blood smear residual lymphocytosis. The increase in BM fat in aplasia is caused
shows characteristic abnormalities. Acute leukemia in children and by increases in the size and number of individual fat cells. “Hot
the elderly can manifest as BM hypocellularity, requiring a careful pockets” of hematopoiesis can be present. The BM tends to contract
search for lymphoblasts or myeloblasts, including phenotypic analysis centripetally with age, and a similar process can be observed in
by flow cytometry. Blood flow cytometry for glycophosphoinositol- pathologic states, so the sternal BM can be more cellular than iliac
anchored proteins should be performed to diagnose PNH (see crest samples.
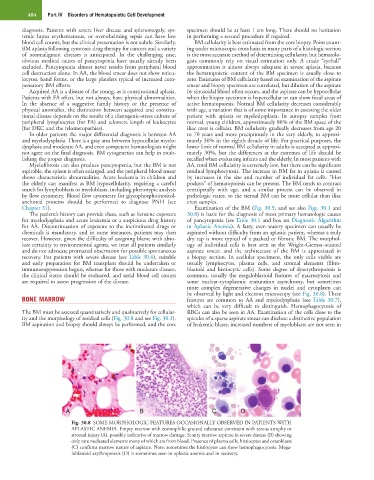
Chapter 31). Examination of the BM (Fig. 30.9, and see also Figs. 30.1 and
The patient’s history can provide clues, such as benzene exposure 30.8) is basic for the diagnosis of most primary hematologic causes
for myelodysplasia and acute leukemia or a suspicious drug history of pancytopenia (see Table 30.1 and box on Diagnostic Algorithm
for AA. Discontinuation of exposure to the incriminated drugs or in Aplastic Anemia). A fatty, even watery specimen can usually be
chemicals is mandatory, and in some instances, patients may then aspirated without difficulty from an aplastic patient, whereas a truly
recover. However, given the difficulty of assigning blame with abso- dry tap is more typical of a packed or fibrotic BM. The morphol-
lute certainty to environmental agents, we treat all patients similarly ogy of individual cells is best seen in the Wright-Giemsa–stained
and do not advocate protracted observation for possible spontaneous aspirate smear, and the architecture of the BM is appreciated in
recovery. For patients with severe disease (see Table 30.6), suitable a biopsy section. In acellular specimens, the only cells visible are
and early preparation for BM transplant should be undertaken or usually lymphocytes, plasma cells, and stromal elements (fibro-
immunosuppression begun, whereas for those with moderate disease, blastoid and histiocytic cells). Some degree of dyserythropoiesis is
the clinical status should be evaluated, and serial blood cell counts common, usually the megaloblastoid features of macrocytosis and
are required to assess progression of the disease. some nuclear-cytoplasmic maturation asynchrony, but sometimes
more complex degenerative changes in nuclei and cytoplasm can
be observed by light and electron microscopy (see Fig. 30.8). These
BONE MARROW features are common to AA and myelodysplasia (see Table 30.7),
which can be very difficult to distinguish. Hemophagocytosis of
The BM must be assessed quantitatively and qualitatively for cellular- RBCs can also be seen in AA. Examination of the cells close to the
ity and the morphology of residual cells (Fig. 30.8 and see Fig. 30.1). spicules of a sparse aspirate smear can disclose a distinctive population
BM aspiration and biopsy should always be performed, and the core of leukemic blasts; increased numbers of myeloblasts are not seen in
A B C D
Fig. 30.8 SOME MORPHOLOGIC FEATURES OCCASIONALLY OBSERVED IN PATIENTS WITH
APLASTIC ANEMIA. Empty marrow with eosinophilic ground substance consistent with serous atrophy or
stromal injury (A), possibly indicative of marrow damage. Scanty marrow aspirate in severe disease (B) showing
only rare nucleated elements many of which are from blood. Presence of plasma cells, histiocytes and osteoblasts
(C) confirms marrow nature of aspirate. Note: sometimes the histiocytes can show hemophagocytosis. Mega-
loblastoid erythropoiesis (D) is sometimes seen in aplastic anemia and in recovery.