Page 606 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 606
Chapter 39 Megaloblastic Anemias 515
Structure of cobalamin Food cobalamin is stable to high-temperature cooking but is
(components and substitutions) readily converted to inactive cobalamin analogues by ascorbic acid.
Cobalamin is exceptionally well stored in tissues in its coenzyme
N NH 2 forms. Of the total-body content of 2 to 5 mg in adults, about 1 mg
is in the liver. There is an obligatory loss of 0.1% per day (1.3 µg)
N N 5′-deoxyadenosyl- regardless of total-body cobalamin content. It takes about 3 to 4 years
O N methyl- to deplete cobalamin stores when dietary cobalamin is abruptly
CH 2 hydroxo- malabsorbed, but it may take longer to develop nutritional cobalamin
cyano-
OH OH deficiency, because of an efficient enterohepatic circulation, which
15
accounts for turnover of 5 to 10 µg/day of cobalamin.
B
C N C C
A N Co + N Absorption
N C Cobamide
D C Cobalamin in food is usually in coenzyme form (5′-deoxyadenosyl-
N cobalamin [adenosylcobalamin] and methylcobalamin), nonspecifi-
CH
2 CH cally bound to proteins (see Fig. 39.2). In the stomach, peptic
CH 2 digestion at low pH is a prerequisite for cobalamin release from food
2 N protein. Once released by proteolysis, cobalamin preferentially
15
CO binds a high-affinity, 150-kDa, cobalamin-binding protein called R
CH 5:6-dimethyl- protein (a haptocorrin) from gastric juice and saliva that has higher
NH 3 - C
O O benziminazole affinity for cobalamin than gastric intrinsic factor (IF). The
CH 2 P cobalamin–R protein (holo-R protein) complex, along with excess
CH O O unbound (apo)-R protein and IF, pass through into the second part
of the duodenum, where pancreatic proteases degrade holo-R and
CH 2 apo-R proteins (but not IF). This results in the transfer of cobalamin
to IF, a 45-kDa glycoprotein with high-affinity binding (K a = 1.5 ×
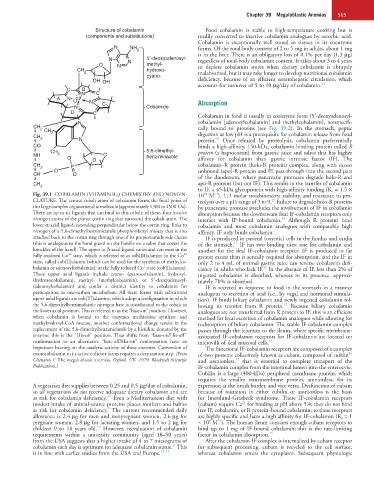
Fig. 39.1 COBALAMIN (VITAMIN B 12) CHEMISTRY AND NOMEN- 10 M ), 1 : 1 molar stoichiometry, stability, and resistance to pro-
10
−1
CLATURE. The central cobalt atom of cobalamin forms the focal point of teolysis over a pH range of 3 to 9. Failure to degrade holo-R proteins
15
this large complex organometallic molecule (approximately 1300 to 1500 Da). by pancreatic protease precludes the involvement of IF in cobalamin
There are up to six ligands that can bind to this cobalt; of these, four involve absorption because the downstream ileal IF-cobalamin receptors only
nitrogen atoms of the planar corrin ring that surround the cobalt atom. The interact with IF-bound cobalamin. Although R proteins bind
15
lower α-axial ligand, extending perpendicular below the corrin ring, links to cobalamin and most cobalamin analogues with comparably high
nitrogen of a 5,6-dimethylbenzimidazole phosphoribosyl moiety that is also affinity, IF only binds cobalamin.
attached back to the corrin ring through one of its propionamide side chains IF is produced in parietal (oxyntic) cells in the fundus and cardia
(this is analogous to the hand guard on the handle on a sabre that covers the of the stomach. IF has two binding sites: one for cobalamin and
15
knuckles of the hand). The upper or β-axial ligand varies and can exist in the another for the ileal IF-cobalamin receptor. IF is produced in far
3+
2+
fully oxidized Co state, which is referred to as cob(III)alamin; in the Co greater excess than is actually required for absorption, and the IF in
state, called cob(II)alamin (which can be used for the synthesis of methylco- only 2 to 4 mL of normal gastric juice can reverse cobalamin defi-
+
balamin or adenosylcobalamin); or the fully reduced Co state (cob[I]alamin). ciency in adults who lack IF. In the absence of IF, less than 2% of
15
These upper axial ligands include cyano- (cyanocobalamin), hydroxyl- ingested cobalamin is absorbed, whereas in its presence, approxi-
(hydroxocobalamin), methyl- (methylcobalamin), or 5′-deoxyadenosyl- mately 70% is absorbed.
(adenosylcobalamin) and confer a distinct identity to cobalamin for IF is secreted in response to food in the stomach in a manner
participation in one-carbon metabolism. All these forms with substituted analogous to secretion of acid (i.e., by vagal and hormonal stimula-
upper axial ligands are cob(III)alamins, which adopt a configuration in which tion). IF binds biliary cobalamin and newly ingested cobalamin fol-
the 5,6-dimethylbenzimidazole nitrogen base is coordinated to the cobalt in lowing its transfer from R protein. Because biliary cobalamin
15
the lower axial position. This is referred to as the “base-on” position. However, analogues are not transferred from R protein to IF, this is an efficient
when cobalamin is bound to the enzymes methionine synthase and method for fecal excretion of cobalamin analogues while allowing for
methylmalonyl-CoA mutase, another conformational change results in the reabsorption of biliary cobalamin. The stable IF-cobalamin complex
replacement of the 5,6-dimethylbenzimidazole by a histidine donated by the passes through the jejunum to the ileum, where specific membrane-
enzyme; this is the “His-on” position. Thus shifts from “base-on/His-off” associated IF-cobalamin receptors for IF-cobalamin are located on
conformation to an alternative “base-off/His-on” conformation have an microvilli of ileal mucosal cells. 15
important bearing on the catalytic activity of these enzymes. Conversion of The functional IF-cobalamin receptors are composed of a complex
cyanocobalamin to its active cofactor forms requires a decyanation step. (From of two proteins collectively known as cubam, composed of cubilin
16
17
Chanarin I: The megaloblastic anemias, Oxford, UK, 1979, Blackwell Scientific and amnionless, that is essential to complete transport of the
Publications.) IF-cobalamin complex from the intestinal lumen into the enterocyte.
Cubilin is a large (400-kDa) peripheral membrane protein, which
requires the smaller transmembrane protein, amnionless, for its
A vegetarian diet supplies between 0.25 and 0.5 µg/day of cobalamin, expression at the brush border, and vice versa. Dysfunction of cubam
so all vegetarians do not receive adequate dietary cobalamin and are because of mutation in either cubilin or amnionless is the basis
7,8
at risk for cobalamin deficiency. Even a Mediterranean diet with for Imerslund-Gräsbeck syndrome. These IF-cobalamin receptors
2+
modest intake of animal-source proteins places mothers and babies (cubam) require Ca for binding at pH above 5.4; they do not bind
9
at risk for cobalamin deficiency. The current recommended daily free IF, cobalamin, or R protein–bound cobalamin; so these receptors
allowance is 2.4 µg for men and nonpregnant women, 2.6 µg for are highly specific and have a high affinity for IF-cobalamin (K a = 1
9
−1
pregnant women, 2.8 µg for lactating women, and 1.5 to 2 µg for × 10 M ). The human ileum contains enough cubam receptors to
10
children 9 to 18 years old. However, reevaluation of cobalamin bind up to 1 mg of IF-bound cobalamin; this is the rate-limiting
requirements within a university community (aged 18–50 years) factor in cobalamin absorption. 15
from the USA suggests that a higher intake of 4 to 7 micrograms of After the cobalamin-IF complex is internalized by cubam receptor
11
cobalamin each day is optimum for adequate cobalamin status. This for subsequent processing, cubam is recycled to the cell surface,
is in line with earlier studies from the USA and Europe. 12–14 whereas cobalamin enters the cytoplasm. Subsequent physiologic