Page 683 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 683
Chapter 41 Pathobiology of Sickle Cell Disease 575
Concentration (g/dL) Fibers Cells 40
20 30 40 30 S/S disease
5 [Polymer] 20
4 “Sickle” 10
B 0 150 200 Number of cells 20 S/C disease
0
3
Log delay time (sec) 2 0 1 C [Polymer] 0 5 10 “Holly leaf” 10 A/S trait
0
40
[Polymer] “Granular” 20 :3 :2 :1 0 1 2 3
0
0.5 0.6 0.7 0.8 Log delay time (sec)
Log [concentration (mM)] 0 0.5 1
A D Time (sec) E
3
Log time to reach 10% (sec) :1
Homogeneous nucleation 2
in 1 sec
Critical 1 Deoxygenation
nucleus
0
:2
:3
0.5 0.6 Instantaneous 0.8
0.7
F Heterogeneous nucleation G Log concentrtion (mM)
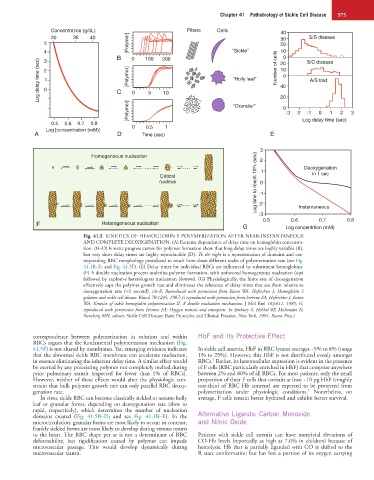
Fig. 41.5 KINETICS OF HEMOGLOBIN S POLYMERIZATION AFTER NEAR-INSTANTANEOUS
AND COMPLETE DEOXYGENATION. (A) Extreme dependence of delay time on hemoglobin concentra-
tion. (B−D) Kinetic progress curves for polymer formation show that long delay times are highly variable (B),
but very short delay times are highly reproducible (D). To the right is a representation of domains and cor-
responding RBC morphology postulated to result from these different scales of polymerization rate (see Fig.
41.1B–E; and Fig. 41.3F). (E) Delay times for individual RBCs are influenced by substituent hemoglobins.
(F) A double nucleation process underlies polymer formation, with unfavored homogeneous nucleation (top)
followed by explosive heterologous nucleation (bottom). (G) Physiologically, the finite rate of deoxygenation
effectively caps the polymer growth rate and eliminates the relevance of delay times that are short relative to
deoxygenation rate (<1 second). (A–E, Reproduced with permission from Eaton WA, Hofrichter J: Hemoglobin S
gelation and sickle cell disease. Blood 70:1245, 1987; F, reproduced with permission from Ferrone FA, Hofrichter J, Eaton
WA: Kinetics of sickle hemoglobin polymerization II. A double nucleation mechanism. J Mol Biol 183:611, 1985; G,
reproduced with permission from Ferrone FA: Oxygen transits and transports. In Embury S, Hebbel RP, Mohandas N,
Steinberg MH, editors: Sickle Cell Disease: Basic Principles and Clinical Practice, New York, 1994, Raven Press.)
correspondence between polymerization in solution and within HbF and Its Protective Effect
RBCs argues that the fundamental polymerization mechanism (Fig.
41.5F) is not altered by membranes. Yet, emerging evidence indicates In sickle cell anemia, HbF in RBC lysates averages ~5% to 8% (range
that the abnormal sickle RBC membrane can accelerate nucleation, 1% to 25%). However, this HbF is not distributed evenly amongst
5
in essence eliminating the inherent delay time. A similar effect would RBCs. Rather, its heterocellular expression is evident in the presence
be exerted by any preexisting polymer not completely melted during of F cells (RBC particularly enriched in HbF) that comprise anywhere
prior pulmonary transit (expected for fewer than 1% of RBCs). between 2% and 80% of all RBCs. For most patients, only the small
However, neither of these effects would alter the physiologic con- proportion of their F cells that contain at least ~10 pg HbF (roughly
straint that bulk polymer growth rate can only parallel RBC deoxy- one-third of RBC Hb content) are expected to be protected from
5
genation rate. polymerization under physiologic conditions. Nonetheless, on
In vitro, sickle RBC can become classically sickled or assume holly average, F cells remain better hydrated and exhibit better survival.
leaf or granular forms, depending on deoxygenation rate (slow to
rapid, respectively), which determines the number of nucleation
domains created (Fig. 41.5B–D; and see Fig. 41.1B–E). In the Alternative Ligands: Carbon Monoxide
microcirculation, granular forms are most likely to occur; in contrast, and Nitric Oxide
frankly sickled forms are most likely to develop during venous return
to the heart. The RBC shape per se is not a determinant of RBC Patients with sickle cell anemia can have nontrivial elevations of
deformability, but rigidification caused by polymer can impede CO-Hb levels (reportedly as high as 7.6% in children) because of
microvascular passage. This would develop dynamically during hemolysis. Hb that is partially liganded with CO is shifted to the
microvascular transit. R state conformation but has lost a portion of its oxygen carrying