Page 722 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 722
Chapter 43 Hemoglobin Variants Associated With Hemolytic Anemia, Altered Oxygen Affinity, and Methemoglobinemias 609
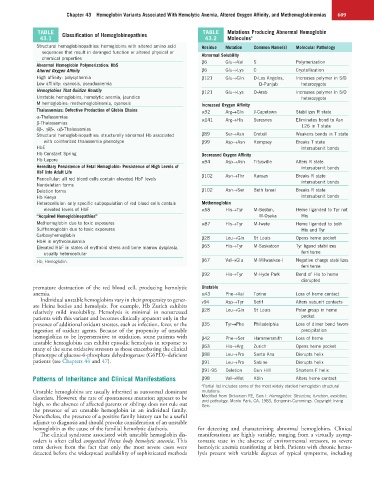
TABLE Classification of Hemoglobinopathies TABLE Mutations Producing Abnormal Hemoglobin
43.1 43.2 Molecules a
Structural hemoglobinopathies: hemoglobins with altered amino acid Residue Mutation Common Name(s) Molecular Pathology
sequences that result in deranged function or altered physical or Abnormal Solubility
chemical properties β6 Glu→Val S Polymerization
Abnormal Hemoglobin Polymerization: HbS
Altered Oxygen Affinity β6 Glu→Lys C Crystallization
High affinity: polycythemia β121 Glu→Gln D-Los Angeles, Increases polymer in S/D
Low affinity: cyanosis, pseudoanemia D-Punjab heterozygote
Hemoglobins That Oxidize Readily β121 Glu→Lys O-Arab Increases polymer in S/O
Unstable hemoglobins, hemolytic anemia, jaundice heterozygote
M hemoglobins: methemoglobinemia, cyanosis Increased Oxygen Affinity
Thalassemias: Defective Production of Globin Chains α92 Arg→Gln J-Capetown Stabilizes R state
α-Thalassemias α141 Arg→His Suresnes Eliminates bond to Asn
β-Thalassemias 126 in T state
δβ-, γδβ-, αβ-Thalassemias
Structural hemoglobinopathies: structurally abnormal Hb associated β89 Ser→Asn Creteil Weakens bonds in T state
with coinherited thalassemia phenotype β99 Asp→Asn Kempsey Breaks T state
HbE intersubunit bonds
Hb Constant Spring Decreased Oxygen Affinity
Hb Lepore α94 Asp→Asn Titusville Alters R state
Hereditary Persistence of Fetal Hemoglobin: Persistence of High Levels of intersubunit bonds
HbF Into Adult Life
Pancellular: all red blood cells contain elevated HbF levels β102 Asn→Thr Kansas Breaks R state
intersubunit bonds
Nondeletion forms
Deletion forms β102 Asn→Ser Beth Israel Breaks R state
Hb Kenya intersubunit bonds
Heterocellular: only specific subpopulation of red blood cells contain Methemoglobin
elevated levels of HbF α58 His→Tyr M-Boston, Heme liganded to Tyr not
“Acquired Hemoglobinopathies” M-Osaka His
Methemoglobin due to toxic exposures α87 His→Tyr M-Iwate Heme liganded to both
Sulfhemoglobin due to toxic exposures His and Tyr
Carboxyhemoglobin β28 Leu→Gln St Louis Opens heme pocket
HbH in erythroleukemia
Elevated HbF in states of erythroid stress and bone marrow dysplasia, β63 His→Tyr M-Saskatoon Tyr ligand stabilizes
usually heterocellular ferriheme
Hb, Hemoglobin. β67 Val→Glu M-Milwaukee-I Negative charge stabilizes
ferriheme
β92 His→Tyr M-Hyde Park Bond of His to heme
disrupted
premature destruction of the red blood cell, producing hemolytic Unstable
anemia. α43 Phe→Val Torino Loss of heme contact
Individual unstable hemoglobins vary in their propensity to gener- v94 Asp→Tyr Setif Alters subunit contacts
ate Heinz bodies and hemolysis. For example, Hb Zurich exhibits
relatively mild insolubility. Hemolysis is minimal in nonstressed β28 Leu→Gln St Louis Polar group in heme
patients with this variant and becomes clinically apparent only in the pocket
presence of additional oxidant stresses, such as infection, fever, or the β35 Tyr→Phe Philadelphia Loss of dimer bond favors
ingestion of oxidant agents. Because of the propensity of unstable precipitation
hemoglobins to be hypersensitive to oxidation, some patients with β42 Phe→Ser Hammersmith Loss of heme
unstable hemoglobins can exhibit episodic hemolysis in response to
many of the same oxidative stressors as those exacerbating the clinical β63 His→Arg Zurich Opens heme pocket
phenotype of glucose-6-phosphate dehydrogenase (G6PD)–deficient β88 Leu→Pro Santa Ana Disrupts helix
patients (see Chapters 44 and 47). β91 Leu→Pro Sabine Disrupts helix
β91-95 Deletion Gun Hill Shortens F helix
Patterns of Inheritance and Clinical Manifestations β98 Val→Met Köln Alters heme contact
a Partial list includes some of the most widely studied hemoglobin structural
Unstable hemoglobins are usually inherited as autosomal dominant mutations.
disorders. However, the rate of spontaneous mutation appears to be Modified from Dickerson RE, Geis I: Hemoglobin: Structure, function, evolution,
and pathology. Menlo Park, CA, 1983, Benjamin-Cummings. Copyright Irving
high, so the absence of affected parents or siblings does not rule out Geis.
the presence of an unstable hemoglobin in an individual family.
Nonetheless, the presence of a positive family history can be a useful
adjunct to diagnosis and should provoke consideration of an unstable
hemoglobin as the cause of the familial hemolytic diathesis. for detecting and characterizing abnormal hemoglobins. Clinical
The clinical syndrome associated with unstable hemoglobin dis- manifestations are highly variable, ranging from a virtually asymp-
orders is often called congenital Heinz body hemolytic anemia. This tomatic state in the absence of environmental stressors, to severe
term derives from the fact that only the most severe cases were hemolytic anemia manifesting at birth. Patients with chronic hemo-
detected before the widespread availability of sophisticated methods lysis present with variable degrees of typical symptoms, including