Page 725 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 725
612 Part V Red Blood Cells
Some patients require transfusions during bouts of severe acute High-affinity Hb
hemolytic anemia. Patients who have significant morbidity because 100 20
of chronic anemia or repeated episodes of severe hemolysis should be 2.5
considered candidates for splenectomy, especially if hypersplenism
has developed. Children with severe hemolysis may require transfu- 5.3
sion support until they are old enough (at least 3 or 4 years of age) 80 16
to undergo splenectomy without unacceptable immunologic com- HbA
promise. Splenectomy is usually effective for abolition or reduction 9.5
of anemia. However, splenectomy should be used only as a last resort Low-affinity Hb
because of the long-term risks of overwhelming sepsis and thrombo- 60 12
sis. Infection often exacerbates hemolysis. Fever should therefore
prompt close monitoring of patients for evidence of hemolysis or O 2 saturation (%) 50 O 2 content (vol %)
infection. Postsplenectomy patients with a hemolytic diathesis are
also afflicted by a hypercoagulable state, probably due to the deranged 40 8
membrane architecture resulting from oxidative damage. They thus
require monitoring for thrombotic events and may need intermittent
or long-term anticoagulant therapy.
20 4
HEMOGLOBINS WITH INCREASED OXYGEN AFFINITY
Efficient oxygen delivery by hemoglobin depends on the sigmoid shape 0 0
of the hemoglobin-oxygen affinity curve. During the transition from 0 20 40 60 80 100
the fully deoxygenated to the fully oxygenated state, the initial oxygen- O pressure (mm Hg)
2
ation steps occur with difficulty. In fact, the act of binding the first
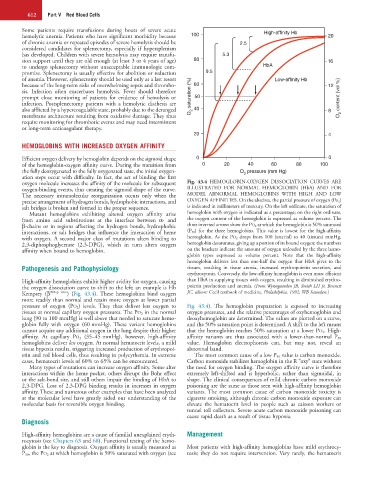
oxygen molecule increases the affinity of the molecule for subsequent Fig. 43.4 HEMOGLOBIN-OXYGEN DISSOCIATION CURVES ARE
oxygen-binding events, thus creating the sigmoid shape of the curve. ILLUSTRATED FOR NORMAL HEMOGLOBIN (HbA) AND FOR
The necessary intramolecular reorganization occurs only when the MODEL ABNORMAL HEMOGLOBINS WITH HIGH AND LOW
precise arrangement of hydrogen bonds, hydrophobic interactions, and OXYGEN AFFINITIES. On the abscissa, the partial pressure of oxygen (Po 2 )
salt bridges is broken and formed in the proper sequence. is indicated in millimeters of mercury. On the left ordinate, the saturation of
Mutant hemoglobins exhibiting altered oxygen affinity arise hemoglobin with oxygen is indicated as a percentage; on the right ordinate,
from amino acid substitutions at the interface between α- and the oxygen content of the hemoglobin is expressed as volume percent. The
β-chains or in regions affecting the hydrogen bonds, hydrophobic three inverted arrows show the PO 2 at which the hemoglobin is 50% saturated
interactions, or salt bridges that influence the interaction of heme (P 50 ) for the three hemoglobins. This value is lowest for the high-affinity
with oxygen. A second major class of mutations alters binding to hemoglobin. As the PO 2 drops from 100 (arterial) to 40 (tissues) mmHg,
2,3-diphosphoglycerate (2,3-DPG), which in turn alters oxygen hemoglobin desaturates, giving up a portion of its bound oxygen; the numbers
affinity when bound to hemoglobin. on the brackets indicate the amount of oxygen unloaded by the three hemo-
globin types expressed as volume percent. Note that the high-affinity
hemoglobin delivers less than one-half the oxygen that HbA gives to the
Pathogenesis and Pathophysiology tissues, resulting in tissue anoxia, increased erythropoietin secretion, and
erythrocytosis. Conversely, the low-affinity hemoglobin is even more efficient
High-affinity hemoglobins exhibit higher avidity for oxygen, causing than HbA in supplying tissues with oxygen, resulting in diminished erythro-
the oxygen dissociation curve to shift to the left; an example is Hb poietin production and anemia. (From Wynngaarden JB, Smith LH Jr, Bennett
Kempsey (β 99Asp→Asn ) (Fig. 43.4). These hemoglobins bind oxygen JC, editors: Cecil textbook of medicine, Philadelphia, 1992, WB Saunders.)
more readily than normal and retain more oxygen at lower partial
pressure of oxygen (PO 2 ) levels. They thus deliver less oxygen to Fig. 43.4). The hemoglobin preparation is exposed to increasing
tissues at normal capillary oxygen pressures. The PO 2 in the normal oxygen pressures, and the relative percentages of oxyhemoglobin and
lung (90 to 100 mmHg) is well above that needed to saturate hemo- deoxyhemoglobin are determined. The values are plotted on a curve,
globin fully with oxygen (60 mmHg). These variant hemoglobins and the 50% saturation point is determined. A shift to the left means
cannot acquire any additional oxygen in the lung despite their higher that the hemoglobin reaches 50% saturation at a lower PO 2 . High-
affinity. At capillary PO 2 (35–45 mmHg), however, high-affinity affinity variants are thus associated with a lower-than-normal P 50
hemoglobins deliver less oxygen. At normal hematocrit levels, a mild value. Hemoglobin electrophoresis can, but may not, reveal an
tissue hypoxia results, triggering increased production of erythropoi- abnormal band.
etin and red blood cells, thus resulting in polycythemia. In extreme The most common cause of a low P 50 value is carbon monoxide.
cases, hematocrit levels of 60% to 65% can be encountered. Carbon monoxide stabilizes hemoglobin in the R “oxy” state without
Many types of mutations can increase oxygen affinity. Some alter the need for oxygen binding. The oxygen affinity curve is therefore
interactions within the heme pocket, others disrupt the Bohr effect extremely left-shifted and is hyperbolic, rather than sigmoidal, in
or the salt-bond site, and still others impair the binding of HbA to shape. The clinical consequences of mild chronic carbon monoxide
2,3-DPG. Loss of 2,3-DPG binding results in increases in oxygen poisoning are the same as those seen with high-affinity hemoglobin
affinity. These and numerous other examples that have been analyzed variants. The most common cause of carbon monoxide toxicity is
at the molecular level have greatly aided our understanding of the cigarette smoking, although chronic carbon monoxide exposure can
molecular basis for reversible oxygen binding. elevate the hematocrit level in people such as caisson workers or
tunnel toll collectors. Severe acute carbon monoxide poisoning can
cause rapid death as a result of tissue hypoxia.
Diagnosis
High-affinity hemoglobins are a cause of familial unexplained eryth- Management
rocytosis (see Chapters 63 and 68). Functional testing of the hemo-
globin is the key to diagnosis. Oxygen affinity is usually measured as Most patients with high-affinity hemoglobins have mild erythrocy-
P 50, the PO 2 at which hemoglobin is 50% saturated with oxygen (see tosis; they do not require intervention. Very rarely, the hematocrit