Page 77 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 77
Chapter 5 Protein Synthesis, Processing, and Trafficking 49
Outer nuclear
membrane
Ribosomes Inner nuclear
mRNA membrane Nucleus
Nuclear
pore
1 5
mRNA
Cytosol
ER signal 8
sequence
2 6 Membrane
Cytosolic 9
protein Targeting Matrix
sequence
Rough ER Peroxisome
Intermembrane
Outer space 7
membrane
Matrix
Inner
3 membrane
Mitochondrion
Golgi apparatus
4a 4b
Plasma membrane Lysosome
Secretory pathway
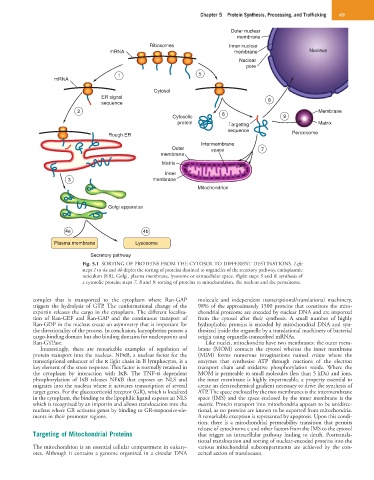
Fig. 5.1 SORTING OF PROTEINS FROM THE CYTOSOL TO DIFFERENT DESTINATIONS. Left:
steps 1 to 4a and 4b depict the sorting of proteins destined to organelles of the secretory pathway, endoplasmic
reticulum (ER), Golgi, plasma membrane, lysosome or extracellular space. Right: steps 5 and 6: synthesis of
a cytosolic protein; steps 7, 8 and 9: sorting of proteins to mitochondrion, the nucleus and the peroxisome.
complex that is transported to the cytoplasm where Ran-GAP molecule and independent transcriptional/translational machinery,
triggers the hydrolysis of GTP. The conformational change of the 98% of the approximately 1500 proteins that constitute the mito-
exportin releases the cargo in the cytoplasm. The different localiza- chondrial proteome are encoded by nuclear DNA and are imported
tion of Ran-GEF and Ran-GAP and the continuous transport of from the cytosol after their synthesis. A small number of highly
Ran-GDP in the nucleus create an asymmetry that is important for hydrophobic proteins is encoded by mitochondrial DNA and syn-
the directionality of the process. In conclusion, karyopherins possess a thesized inside the organelle by a translational machinery of bacterial
cargo-binding domain but also binding domains for nucleoporins and origin using organelle-transcribed mRNAs.
Ran-GTPase. Like nuclei, mitochondria have two membranes: the outer mem-
Interestingly, there are remarkable examples of regulation of brane (MOM) contacts the cytosol whereas the inner membrane
protein transport into the nucleus. NFκB, a nuclear factor for the (MIM) forms numerous invaginations named cristae where the
transcriptional enhancer of the κ light chain in B lymphocytes, is a enzymes that synthesize ATP through reactions of the electron
key element of the stress response. This factor is normally retained in transport chain and oxidative phosphorylation reside. Where the
the cytoplasm by interaction with IκB. The TNF-α dependent MOM is permeable to small molecules (less than 5 kDa) and ions,
phosphorylation of IκB releases NFκB that exposes an NLS and the inner membrane is highly impermeable, a property essential to
migrates into the nucleus where it activates transcription of several create an electrochemical gradient necessary to drive the synthesis of
target genes. For the glucocorticoid receptor (GR), which is localized ATP. The space enclosed by the two membranes is the intermembrane
in the cytoplasm, the binding to the lipophilic ligand exposes an NLS space (IMS) and the space enclosed by the inner membrane is the
which is recognized by an importin and allows translocation into the matrix. Protein transport into mitochondria appears to be unidirec-
nucleus where GR activates genes by binding to GR-responsive-ele- tional, as no proteins are known to be exported from mitochondria.
ments in their promoter regions. A remarkable exception is represented by apoptosis. Upon this condi-
tion, there is a mitochondrial permeability transition that permits
release of cytochrome c and other factors from the IMS to the cytosol
Targeting of Mitochondrial Proteins that trigger an intracellular pathway leading to death. Posttransla-
tional translocation and sorting of nuclear-encoded proteins into the
The mitochondrion is an essential cellular compartment in eukary- various mitochondrial subcompartments are achieved by the con-
otes. Although it contains a genome organized in a circular DNA certed action of translocases.