Page 79 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 79
Chapter 5 Protein Synthesis, Processing, and Trafficking 51
COTRANSLATIONAL PROTEIN TRANSLOCATION INTO to the cell surface. In contrast, proteins that have specific targeting
signals may be localized to the lumen of the ER, the Golgi compart-
THE ENDOPLASMIC RETICULUM ment, or lysosomes. Other proteins that reside in membranes of the
cell contain topologic sequences called transmembrane domains that
The ER is an extensive membranous network that is continuous with consist of ~20 largely apolar amino acids. When a transmembrane
the outer nuclear membrane and is the site for the synthesis of the domain enters the translocon, the polypeptide is released laterally
massive amounts of lipid and protein used to build the membranes from the Sec61 channel into the lipid bilayer. Membrane proteins
of most cellular organelles. The ER comprises three interconnected can assume different topologies according to the number and type of
domains: rough ER, smooth ER, and ER exit sites. The rough ER is TM domains.
so called because it is studded with bound ribosomes that are actively
synthesizing proteins. Cells specialized in protein secretion, such as
cells of the exocrine glands and plasma cells are rich in rough ER. PROTEIN TRAFFICKING WITHIN THE
The smooth ER lacks ribosomes, is not very abundant in most cells SECRETORY PATHWAY
(except hepatocytes), and is thought to be the site of lipid biosynthesis
and of cytochrome P450–mediated detoxification reactions. Finally, Proteins that enter the ER are transported towards the plasma
ER exit sites are specialized areas of the ER membrane where transport membrane through the secretory pathway (Fig. 5.4). Specific signals
cargo is packaged into transport vesicles en route to the Golgi cause resident proteins to be retained in the ER, Golgi or plasma
apparatus. membrane. Proteins may also be targeted from the Golgi compart-
Nascent secretory proteins are marked for translocation into ment to lysosomes or from the plasma membrane to endosomes (see
the ER by the presence of an amino-terminal signal sequence (see Fig. 5.4, pathways 8 and 9). Initially the study of this complex protein
Table 5.1). This sequence has a length of about 15–30 amino acids trafficking took advantage of the use of yeast genetics to isolate
and displays no conservation of amino acid sequence, although it temperature-sensitive mutants (sec) that were defective at different
contains a hydrophobic core flanked by polar residues that prefer- stages of the secretory pathway. The subsequent characterization of
entially have a short side chain in proximity to the cleavage site. As SEC genes, thanks to the advent of DNA recombinant techniques,
the signal sequence emerges from the ribosome, it is recognized by made possible the isolation of the counterparts in mammalian cells
the signal recognition particle (SRP), a ribonucleoprotein, and this and the beginning of molecular and biochemical investigation of
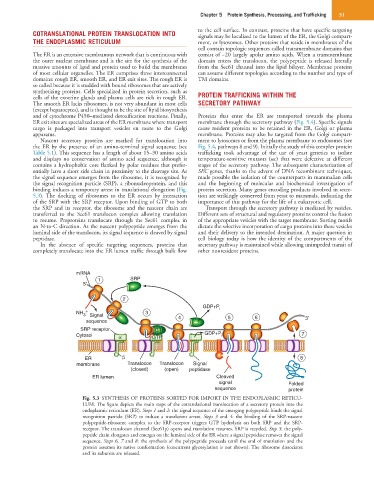
binding induces a temporary arrest in translational elongation (Fig. protein secretion. Many genes encoding products involved in secre-
5.3). The docking of ribosomes to the ER occurs by interaction tion are strikingly conserved from yeast to mammals, indicating the
of the SRP with the SRP receptor. Upon binding of GTP to both importance of this pathway for the life of a eukaryotic cell.
the SRP and its receptor, the ribosome and the nascent chain are Transport through the secretory pathway is mediated by vesicles.
transferred to the Sec61 translocon complex allowing translation Different sets of structural and regulatory proteins control the fusion
to resume. Preproteins translocate through the Sec61 complex in of the appropriate vesicles with the target membrane. Sorting motifs
an N-to-C direction. As the nascent polypeptide emerges from the dictate the selective incorporation of cargo proteins into these vesicles
luminal side of the translocon, its signal sequence is cleaved by signal and their delivery to the intended destination. A major question in
peptidase. cell biology today is how the identity of the compartments of the
In the absence of specific targeting sequences, proteins that secretory pathway is maintained while allowing unimpeded transit of
completely translocate into the ER lumen traffic through bulk flow other nonresident proteins.
mRNA
1 SRP
5′
2
GDP+P i
+
NH 3 Signal 3
sequence 4 5 6 3′
SRP receptor GTP
Cytosol α GTP GDP+P i 7
ER β 8
membrane Translocon Translocon Signal
(closed) (open) peptidase
ER lumen Cleaved
signal Folded
sequence protein
Fig. 5.3 SYNTHESIS OF PROTEINS SORTED FOR IMPORT IN THE ENDOPLASMIC RETICU-
LUM. The figure depicts the main steps of the cotranslational translocation of a secretory protein into the
endoplasmic reticulum (ER). Steps 1 and 2: the signal sequence of the emerging polypeptide binds the signal
recognition particle (SRP) to induce a translation arrest. Steps 3 and 4: the binding of the SRP-nascent
polypeptide-ribosome complex to the SRP-receptor triggers GTP hydrolysis on both SRP and the SRP-
receptor. The translocon channel (Sec61p) opens and translation resumes. SRP is recycled. Step 5: the poly-
peptide chain elongates and emerges on the luminal side of the ER where a signal peptidase removes the signal
sequence. Steps 6, 7 and 8: the synthesis of the polypeptide proceeds until the end of translation and the
protein assumes its native conformation (concurrent glycosylation is not shown). The ribosome dissociates
and its subunits are released.