Page 872 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 872
Chapter 54 Infectious Mononucleosis and Other Epstein-Barr Virus–Associated Diseases 755
of MS is the subject of active investigation. Based on findings from HIV infection, and immunosuppression in SOT recipients (Fig. 54.6,
20
these studies, EBV-targeted approaches might be explored in A, B) or HSCT. Besides EBV and a dysfunctional cellular immune
the future. system, genetic alterations in B cells have also been implicated in
the pathogenesis of posttransplant LPD, especially in SOT recipi-
ents, including microsatellite instability, DNA hypermethylation,
Epstein-Barr Virus–Associated Malignancies aberrant somatic hypermutation, and mutations in specific genes
such as MYCC, BCL-6, N-ras, and p53. Most cases of EBV-LPD
Over the past decades, EBV has been associated with a heterogeneous are lymphomas of B-cell origin, histologic high-grade NHL of the
15
group of malignancies. Each year 200,000 cases of EBV-positive immunoblastic or undifferentiated large cell type that respond poorly
malignancies are diagnosed worldwide, with gastric carcinoma being to cytotoxic therapy. In the setting of SOT, the reported incidence
the most common, followed by NPC, and lymphoma. Although of EBV-LPD ranges from 1% to 25%, with the highest risk in sero-
there is strong circumstantial evidence linking EBV to these malig- negative recipients, patients receiving intensive immunosuppressive
nancies, the potential causative relationship between EBV and these therapy, and patients receiving grafts with a high lymphoid content.
tumors remains to be firmly established. The following section After HSCT the incidence of EBV-LPD varies with the transplant
focuses on EBV-LPD, HD, NHL (including BL), and NPC regimen and may be as high as 25%. Risk factors for the development
(Figs. 54.6 and 54.7). All EBV-associated malignancies are associated of EBV-LPD include the use of stem cells from an HLA-mismatched
with viral latency, and spontaneous viral replication occurs at a very family member or closely HLA-matched unrelated donor, T-cell
low frequency. Because antiviral agents, like acyclovir, only prevent depletion of the donor cells, intensive immunosuppression, and an
viral replication and do not affect latency, these agents are of limited underlying diagnosis of primary immunodeficiency. The incidence is
therapeutic value. much lower when methods that also deplete B cells are employed. The
onset of EBV-LPD seems to be preceded by a large increase in virus
load as well as the proliferation of EBV-infected B cells. Frequent
Lymphoproliferative Disease monitoring of the EBV-DNA load in peripheral blood is a valuable
diagnostic test for early detection of EBV-LPD after HSCT or SOT.
EBV-LPD develops in patients with congenital or acquired immu- However, it remains a subject of debate which is the optimal sample
nodeficiencies, including severe combined immunodeficiency, XLP, (whole blood, isolated peripheral blood mononuclear cells, plasma)
A B C D E F
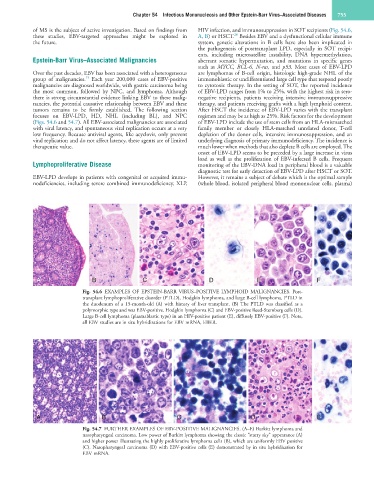
Fig. 54.6 EXAMPLES OF EPSTEIN-BARR VIRUS–POSITIVE LYMPHOID MALIGNANCIES. Post-
transplant lymphoproliferative disorder (PTLD), Hodgkin lymphoma, and large B-cell lymphoma. PTLD in
the duodenum of a 15-month-old (A) with history of liver transplant. (B) The PTLD was classified as a
polymorphic type and was EBV-positive. Hodgkin lymphoma (C) and EBV-positive Reed-Sternberg cells (D).
Large B-cell lymphoma (plasmablastic type) in an HIV-positive patient (E), diffusely EBV-positive (F). Note,
all EBV studies are in situ hybridizations for EBV mRNA, EBER.
G
A B C D E
Fig. 54.7 FURTHER EXAMPLES OF EBV-POSITIVE MALIGNANCIES. (A–E) Burkitt lymphoma and
nasopharyngeal carcinoma. Low power of Burkitt lymphoma showing the classic “starry sky” appearance (A)
and higher power illustrating the highly proliferative lymphoma cells (B), which are uniformly EBV positive
(C). Nasopharyngeal carcinoma (D) with EBV-positive cells (E) demonstrated by in situ hybridization for
EBV mRNA.