Page 92 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 92
Chapter 6 Protein Architecture: Relationship of Form and Function 63
Bound peptide
TCR
Peptide
MHC
Heavy chain Light chain
A B
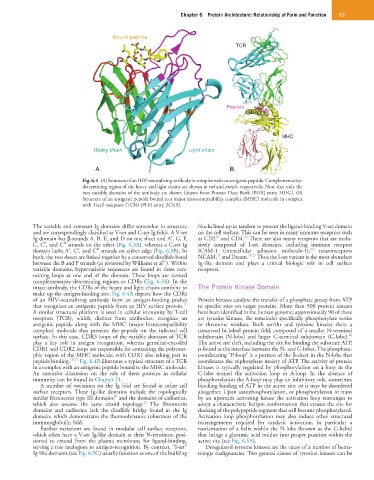
Fig. 6.4 (A) Structure of an HIV-neutralizing antibody in complex with an antigenic peptide. Complementarity-
determining region of the heavy and light chains are shown in red and purple, respectively. Note that only the
two variable domains of the antibody are shown (drawn from Protein Data Bank [PDB] entry 3IDG). (B)
Structure of an antigenic peptide bound to a major histocompatibility complex (MHC) molecule in complex
with T-cell receptors (TCR) (PDB entry 2CKB).
The variable and constant Ig domains differ somewhat in structure blocks lined up in tandem to present the ligand-binding V-set domain
and are correspondingly classified as V-set and C-set Ig folds. A V-set on the cell surface. This can be seen in many immune receptors such
22
23
Ig domain has β-strands A, B, E, and D on one sheet and A′, G, F, as CD2 and CD4. There are also many receptors that are exclu-
C, C′, and C″ strands on the other (Fig. 6.3A), whereas a C-set Ig sively composed of I-set domains, including immune receptor
24
domain lacks A′, C′, and C″ strands on either edge (Fig. 6.3B). In ICAM-1 (intercellular adhesion molecule-1), neuroreceptors
25
both, the two sheets are linked together by a conserved disulfide bond NCAM, and Dscam. 26,27 Thus the I-set variant is the most abundant
16
between the B and F strands (as reviewed by Williams et al ). Within Ig-like domain and plays a critical biologic role in cell surface
variable domains, hypervariable sequences are found in three con- receptors.
necting loops at one end of the domain. These loops are termed
complementarity-determining regions or CDRs (Fig. 6.3A). In the
intact antibody, the CDRs of the heavy and light chains combine to The Protein Kinase Domain
make up the antigen-binding site. Fig. 6.4A depicts how the CDRs
of an HIV-neutralizing antibody form an antigen-binding pocket Protein kinases catalyze the transfer of a phosphate group from ATP
17
that recognizes an antigenic peptide from an HIV surface protein. to specific sites on target proteins. More than 500 protein kinases
A similar structural platform is used in cellular immunity by T-cell have been identified in the human genome; approximately 90 of these
receptors (TCR), which, distinct from antibodies, recognize an are tyrosine kinases, the remainder specifically phosphorylate serine
antigenic peptide along with the MHC (major histocompatibility or threonine residues. Both ser/thr and tyrosine kinases share a
complex) molecule that presents the peptide on the infected cell conserved bi-lobed protein fold, composed of a smaller N-terminal
28
surface. In this case, CDR3 loops of the variable domains of TCR subdomain (N-lobe) and larger C-terminal subdomain (C-lobe).
play a key role in antigen recognition, whereas germline-encoded The active site cleft, including the site for binding the substrate ATP,
CDR1 and CDR2 loops are responsible for contacting the polymor- is found at the interface between the N- and C-lobes. The phosphate-
phic region of the MHC molecule, with CDR1 also taking part in coordinating “P-loop” is a portion of the β-sheet in the N-lobe that
peptide binding. 18,19 Fig. 6.4B illustrates a typical structure of a TCR coordinates the triphosphate moiety of ATP. The activity of protein
in a complex with an antigenic peptide bound to the MHC molecule. kinases is typically regulated by phosphorylation on a loop in the
An extensive discussion on the role of these proteins in cellular C-lobe termed the activation loop or A-loop. In the absence of
immunity can be found in Chapter 21. phosphorylation the A-loop may play an inhibitory role, sometimes
A number of variations on the Ig fold are found in other cell blocking binding of ATP in the active site; or it may be disordered
surface receptors. These Ig-like domains include the topologically altogether. Upon autophosphorylation, or phosphorylation in trans
20
similar fibronectin type III domains and the domains of cadherins, by an upstream activating kinase the activation loop rearranges to
21
which also assume the same strand topology. The fibronectin adopt a characteristic hairpin conformation that creates the site for
domains and cadherins lack the disulfide bridge found in the Ig docking of the polypeptide segment that will become phosphorylated.
domain, which demonstrates the thermodynamic robustness of the Activation loop phosphorylation may also induce other structural
immunoglobulin fold. rearrangements required for catalytic activation, in particular a
Further variations are found in modular cell surface receptors, reorientation of a helix within the N-lobe (known as the C-helix)
which often have a V-set Ig-like domain at their N-terminus, posi- that brings a glutamic acid residue into proper position within the
tioned to extend from the plasma membrane for ligand-binding, active site (see Fig. 6.5A).
serving a role analogous to antigen-recognition. By contrast, “I-set” Deregulated tyrosine kinases are the cause of a number of hema-
Ig-like domains (see Fig. 6.3C) usually function as one of the building tologic malignancies. Two general classes of tyrosine kinases can be