Page 94 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 94
Chapter 6 Protein Architecture: Relationship of Form and Function 65
Inactive Active Trx-like domain
Extracellular
Cytoplasmic TM6
TM5 TM6 TM5
Gβγ
Gα s
UQ
Membrane TM4 TM5
TM1
TM3
TM2
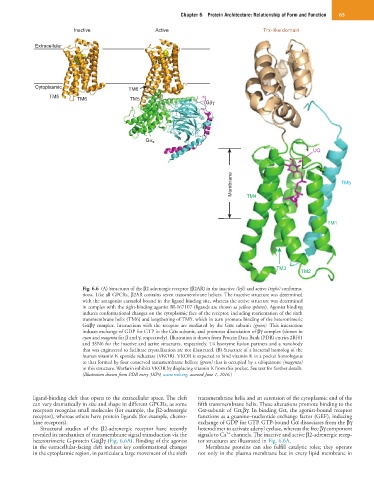
Fig. 6.6 (A) Structures of the β2 adrenergic receptor (β2AR) in the inactive (left) and active (right) conforma-
tions. Like all GPCRs, β2AR contains seven transmembrane helices. The inactive structure was determined
with the antagonist carazolol bound in the ligand binding site, whereas the active structure was determined
in complex with the tight-binding agonist BI-167107 (ligands are shown as yellow spheres). Agonist binding
induces conformational changes on the cytoplasmic face of the receptor, including reorientation of the sixth
transmembrane helix (TM6) and lengthening of TM5, which in turn promote binding of the heterotrimeric
Gαβγ complex. Interactions with the receptor are mediated by the Gαs subunit (green). This interaction
induces exchange of GDP for GTP in the Gαs subunit, and promotes dissociation of βγ complex (shown in
cyan and magenta for β and γ, respectively). Illustration is drawn from Protein Data Bank (PDB) entries 2RH1
and 3SN6 for the inactive and active structures, respectively. T4 lysozyme fusion partners and a nanobody
that was engineered to facilitate crystallization are not illustrated. (B) Structure of a bacterial homolog of the
human vitamin K epoxide reductase (VKOR). VKOR is expected to bind vitamin K in a pocket homologous
to that formed by four conserved transmembrane helices (green) that is occupied by a ubiquinone (magenta)
in this structure. Warfarin inhibits VKOR by displacing vitamin K from this pocket. See text for further details.
(Illustration drawn from PDB entry 3KP9, www.rcsb.org, accessed June 1, 2016.)
ligand-binding cleft that opens to the extracellular space. The cleft transmembrane helix and an extension of the cytoplasmic end of the
can vary dramatically in size and shape in different GPCRs, as some fifth transmembrane helix. These alterations promote binding to the
receptors recognize small molecules (for example, the β2-adrenergic Gα-subunit of Gα sβγ. In binding Gα, the agonist-bound receptor
receptor), whereas others have protein ligands (for example, chemo- functions as a guanine–nucleotide exchange factor (GEF), inducing
kine receptors). exchange of GDP for GTP. GTP-bound Gα dissociates from the βγ
Structural studies of the β2-adrenergic receptor have recently heterodimer to activate adenyl cyclase, whereas the free βγ component
2+
revealed its mechanism of transmembrane signal transduction via the signals to Ca channels. The inactive and active β2-adrenergic recep-
heterotrimeric G-protein Gα s βγ (Fig. 6.6A). Binding of the agonist tor structures are illustrated in Fig. 6.6A.
in the extracellular-facing cleft induces key conformational changes Membrane proteins can also fulfill catalytic roles; they operate
in the cytoplasmic region, in particular a large movement of the sixth not only in the plasma membrane but in every lipid membrane in