Page 93 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 93
64 Part I Molecular and Cellular Basis of Hematology
SH3
N-lobe
C-helix
P-loop
Activation
loop
C-lobe SH2
Kinase
A B
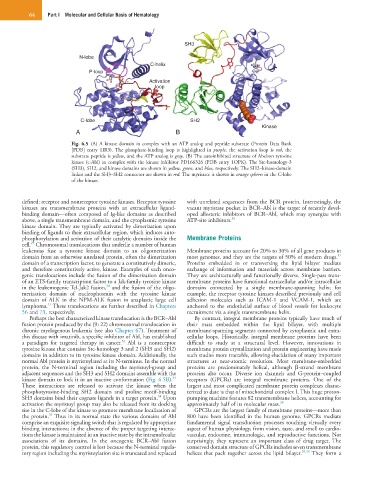
Fig. 6.5 (A) A kinase domain in complex with an ATP analog and peptide substrate (Protein Data Bank
[PDB] entry 1IR3). The phosphate-binding loop is highlighted in purple, the activation loop is red, the
substrate peptide is yellow, and the ATP analog is gray. (B) The autoinhibited structure of Abelson tyrosine
kinase (c-Abl) in complex with the kinase inhibitor PD166326 (PDB entry 1OPK). The Src-homology-3
(SH3), SH2, and kinase domains are shown in yellow, green, and blue, respectively. The SH2–kinase-domain
linker and the SH3–SH2 connector are shown in red. The myristate is shown in orange spheres in the C-lobe
of the kinase.
defined: receptor and nonreceptor tyrosine kinases. Receptor tyrosine with unrelated sequences from the BCR protein. Interestingly, the
kinases are transmembrane proteins with an extracellular ligand- vacant myristate pocket in BCR–Abl is the target of recently devel-
binding domain—often composed of Ig-like domains as described oped allosteric inhibitors of BCR–Abl, which may synergize with
above, a single transmembrane domain, and the cytoplasmic tyrosine ATP-site inhibitors. 36
kinase domain. They are typically activated by dimerization upon
binding of ligands to their extracellular region, which induces auto-
phosphorylation and activation of their catalytic domains inside the Membrane Proteins
29
cell. Chromosomal translocations that underlie a number of human
leukemias fuse a tyrosine kinase domain to an oligomerization Membrane proteins account for 20% to 30% of all gene products in
37
domain from an otherwise unrelated protein, often the dimerization most genomes, and they are the targets of 50% of modern drugs.
domain of a transcription factor, to generate a constitutively dimeric, Proteins embedded in or transversing the lipid bilayer mediate
and therefore constitutively active, kinase. Examples of such onco- exchange of information and materials across membrane barriers.
genic translocations include the fusion of the dimerization domain They are architecturally and functionally diverse. Single-pass trans-
of an ETS-family transcription factor to a Jak-family tyrosine kinase membrane proteins have functional extracellular and/or intracellular
30
in the leukemogenic Tel-Jak2 fusion, and the fusion of the oligo- domains connected by a single membrane-spanning helix; for
merization domain of nucleophosmin with the tyrosine kinase example, the receptor tyrosine kinases described previously and cell
domain of ALK in the NPM-ALK fusion in anaplastic large cell adhesion molecules such as ICAM-1 and VCAM-1, which are
31
lymphoma. These translocations are further described in Chapters anchored to the endothelial surface of blood vessels for leukocyte
56 and 73, respectively. recruitment via a single transmembrane helix.
Perhaps the best characterized kinase translocation is the BCR–Abl By contrast, integral membrane proteins typically have much of
fusion protein produced by the (9 : 22) chromosomal translocation in their mass embedded within the lipid bilayer, with multiple
chronic myelogenous leukemia (see also Chapter 67). Treatment of membrane-spanning segments connected by cytoplasmic and extra-
this disease with imatinib, a specific inhibitor of Abl, has established cellular loops. Historically, integral membrane proteins have been
32
a paradigm for targeted therapy in cancer. Abl is a nonreceptor difficult to study at a structural level. However, innovations in
tyrosine kinase that contains Src-homology 3 and 2 (SH3 and SH2) membrane protein crystallization and protein engineering have made
domains in addition to its tyrosine kinase domain. Additionally, the such studies more tractable, allowing elucidation of many important
normal Abl protein is myristoylated at its N-terminus. In the normal structures at near-atomic resolution. Most membrane-embedded
protein, the N-terminal region including the myristoyl-group and proteins are predominately helical, although β-strand membrane
adjacent sequences and the SH3 and SH2 domains assemble with the proteins also occur. Diverse ion channels and G-protein–coupled
33
kinase domain to lock it in an inactive conformation (Fig. 6.5B). receptors (GPCRs) are integral membrane proteins. One of the
These interactions are released to activate the kinase when the largest and most complicated membrane protein complexes charac-
phosphotyrosine-binding SH2 domain and proline motif-binding terized to date is that of mitochondrial complex I. This huge proton-
34
SH3 domains bind their cognate ligands in a target protein. Upon pumping machine features 82 transmembrane helices, accounting for
activation the myristoyl group may also be released from its docking approximately half of its molecular mass. 38
site in the C-lobe of the kinase to promote membrane localization of GPCRs are the largest family of membrane proteins—more than
35
the protein. Thus in its normal state the various domains of Abl 800 have been identified in the human genome. GPCRs mediate
comprise an exquisite signaling switch that is regulated by appropriate fundamental signal transduction processes touching virtually every
binding interactions; in the absence of the proper targeting interac- aspect of human physiology, from vision, taste, and smell to cardio-
tions the kinase is maintained in an inactive state by the intramolecular vascular, endocrine, immunologic, and reproductive functions. Not
associations of its domains. In the oncogenic BCR–Abl fusion surprisingly, they represent an important class of drug target. The
protein, this regulatory control is lost because the N-terminal regula- conserved domain structure of GPCRs includes seven transmembrane
tory region including the myristoylation site is truncated and replaced helices that pack together across the lipid bilayer. 39,40 They form a