Page 970 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 970
Chapter 57 Pharmacology and Molecular Mechanisms of Antineoplastic Agents for Hematologic Malignancies 853
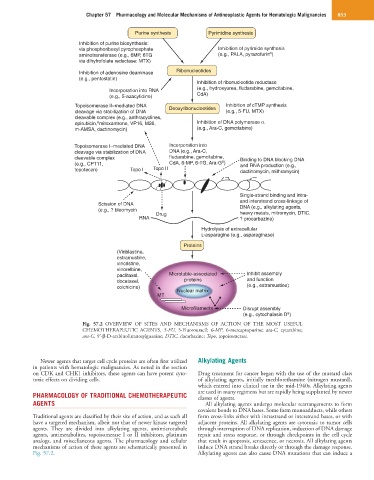
Purine synthesis Pyrimidine synthesis
Inhibition of purine biosynthesis:
via phosphoribosyl pyrophosphate Inhibition of pyrimide synthesis
a
aminotransferase (e.g., 6MP, 6TG (e.g., PALA, pyrazofurin )
via dihyfrofolate reductase: MTX)
Inhibition of adenosine deaminase Ribonucleotides
(e.g., pentostatin)
Inhibition of ribonucleotide reductase
(e.g., hydroxyurea, fludarabine, gemcitabine,
Incorporation into RNA
(e.g., 5-azacytidine) CdA)
Topoisomerase II–mediated DNA Deoxyribonucleotides Inhibition of cTMP synthesis
cleavage via stabilization of DNA (e.g., 5-FU, MTX)
cleavable complex (e.g., anthracyclines,
a
epirubicin, mitoxantrone, VP16, M26, Inhibition of DNA polymerase α
m-AMSA, dactinomycin) (e.g., Ara-C, gemcitabine)
Topoisomerase I–mediated DNA Incorporation into
cleavage via stabilization of DNA DNA (e.g., Ara-C,
cleavable complex fludarabine, gemcitabine, Binding to DNA blocking DNA
a
(e.g., CPT11, CdA, 6-MP, 6-TG, Ara-G ) and RNA production (e.g.,
topotecan) Topo I Topo II dactinomycin, mithramycin)
Single-strand binding and intra-
and interstrand cross-linkage of
Scission of DNA
(e.g., ? bleomycin DNA (e.g., alkylating agents,
heavy metals, mitromycin, DTIC.
RNA Drug ? procarbazine)
Hydrolysis of extracellular
L-asparagine (e.g., asparaginase)
Proteins
(Vinblastine,
estramustine,
vincristine,
vinorelbine,
paclitaxel, Microtuble-associated Inhibit assembly
docetaxel, proteins and function
colchicine) (e.g., estramustine)
Nuclear matrix
MT
Microfilaments Disrupt assembly
a
(e.g., cytochalasin D )
Fig. 57.2 OVERVIEW OF SITES AND MECHANISMS OF ACTION OF THE MOST USEFUL
CHEMOTHERAPEUTIC AGENTS. 5-FU, 5-Fluorouracil; 6-MP, 6-mercaptopurine; ara-C, cytarabine;
ara-G, 9′-β-D-arabinofuranosylguanine; DTIC, dacarbazine; Topo, topoisomerase.
Newer agents that target cell cycle proteins are often first utilized Alkylating Agents
in patients with hematologic malignancies. As noted in the section
on CDK and CHK1 inhibitors, these agents can have potent cyto- Drug treatment for cancer began with the use of the mustard class
toxic effects on dividing cells. of alkylating agents, initially mechlorethamine (nitrogen mustard),
which entered into clinical use in the mid-1940s. Alkylating agents
PHARMACOLOGY OF TRADITIONAL CHEMOTHERAPEUTIC are used in many regimens but are rapidly being supplanted by newer
classes of agents.
AGENTS All alkylating agents undergo molecular rearrangements to form
covalent bonds to DNA bases. Some form monoadducts, while others
Traditional agents are classified by their site of action, and as such all form cross-links either with intrastrand or interstrand bases, or with
have a targeted mechanism, albeit not that of newer kinase targeted adjacent proteins. All alkylating agents are cytotoxic to tumor cells
agents. They are divided into alkylating agents, antimicrotubule through interruption of DNA replication, induction of DNA damage
agents, antimetabolites, topoisomerase I or II inhibitors, platinum repair and stress response, or through checkpoints in the cell cycle
analogs, and miscellaneous agents. The pharmacology and cellular that result in apoptosis, senescence, or necrosis. All alkylating agents
mechanisms of action of these agents are schematically presented in induce DNA strand breaks directly or through the damage response.
Fig. 57.2. Alkylating agents can also cause DNA mutations that can induce a