Page 971 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 971
854 Part VII Hematologic Malignancies
cytotoxic response or can alter proteins, leading to immune responses Cyclophosphamide is a nitrogen mustard with a ringed struc-
to novel protein sequences. ture off the end-chloroethyl backbone that decreases spontaneous
decomposition (see Fig. 57.3). Enzymatic activation is required
through multifunction P450 enzymes in the liver. Phenobarbital
Mechanisms of Resistance and corticosteroids may alter activation. The bioavailability of
cyclophosphamide orally and intravenously is quite similar, although
Efficient removal of the DNA adducts reduces the lesion burden, most use of the drug is by bolus IV injection. Cyclophosphamide is
while loss of DNA damage recognition can invoke damage tolerance, detoxified through oxidation to 4-keto-cyclophosphamide and car-
as in the case of loss of MMR and temozolomide tolerance. Detoxify- boxyphosphamide by aldehyde dehydrogenase. Cyclophosphamide
ing enzymes can serve as acceptor molecules for alkylation, alter the is used in doses as small as 50–100 mg/day orally (PO) and in
2
2
agent prior to DNA attack, or metabolize the parent compound. Loss bolus doses of 400–700 mg/m for solid tumors and 750 mg/m
of TP53 results in loss of cell cycle checkpoint induction of DNA in combination with doxorubicin and vincristine and prednisone as
repair signals. Increased AKT signaling promotes cell proliferation to part of the CHOP regimen for NHLs, or alone or with bortezomib
compensate for the DNA damage response-associated toxicity. for patients with myeloma. It is also used at doses of up to 60 mg/
kg/day for 4 days in autologous and allogeneic bone marrow (BM)
transplantation protocols. Cyclophosphamide is used in numerous
Nitrogen Mustard treatment protocols for NHLs and high-dose therapy regimens
designed to eradicate tumor and BM in patients with lymphomas
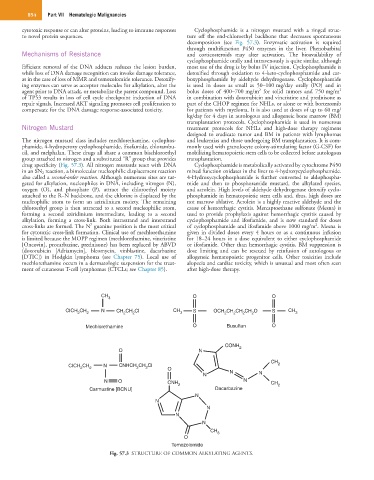
The nitrogen mustard class includes mechlorethamine, cyclophos- and leukemias and those undergoing BM transplantation. It is com-
phamide, 4-hydroperoxy cyclophosphamide, ifosfamide, chlorambu- monly used with granulocyte colony-stimulating factor (G-CSF) for
cil, and melphalan. These drugs all share a common bischloroethyl mobilizing hematopoietic stem cells to be collected before autologous
group attached to nitrogen and a substituted “R” group that provides transplantation.
drug specificity (Fig. 57.3). All nitrogen mustards react with DNA Cyclophosphamide is metabolically activated by cytochrome P450
in an SN 2 reaction, a bimolecular nucleophilic displacement reaction mixed function oxidases in the liver to 4-hydroxycyclophosphamide.
also called a second-order reaction. Although numerous sites are tar- 4-Hydroxycyclophosphamide is further converted to aldophospha-
geted for alkylation, nucleophiles in DNA, including nitrogen (N), mide and then to phosphoramide mustard, the alkylated species,
oxygen (O), and phosphate (P), attract the chloroethyl moiety and acrolein. High levels of aldehyde dehydrogenase detoxify cyclo-
attached to the R–N backbone, and the chlorine is displaced by the phosphamide in hematopoietic stem cells and, thus, high doses are
nucleophilic atom to form an aziridinium moiety. The remaining not marrow ablative. Acrolein is a highly reactive aldehyde and the
chloroethyl group is then attracted to a second nucleophilic atom, cause of hemorrhagic cystitis. Mercaptoethane sulfonate (Mesna) is
forming a second aziridinium intermediate, leading to a second used to provide prophylaxis against hemorrhagic cystitis caused by
alkylation, forming a cross-link. Both intrastrand and interstrand cyclophosphamide and ifosfamide, and is now standard for doses
2
7
cross-links are formed. The N guanine position is the most critical of cyclophosphamide and ifosfamide above 1000 mg/m . Mesna is
for cytotoxic cross-link formation. Clinical use of mechlorethamine given in divided doses every 4 hours or as a continuous infusion
is limited because the MOPP regimen (mechlorethamine, vincristine for 18–24 hours in a dose equivalent to either cyclophosphamide
[Oncovin], procarbazine, prednisone) has been replaced by ABVD or ifosfamide. Other than hemorrhagic cystitis, BM suppression is
(doxorubicin [Adriamycin], bleomycin, vinblastine, dacarbazine dose limiting and can be rescued by reinfusion of autologous or
[DTIC]) in Hodgkin lymphoma (see Chapter 75). Local use of allogeneic hematopoietic progenitor cells. Other toxicities include
mechlorethamine occurs in a dermatologic suspension for the treat- alopecia and cardiac toxicity, which is unusual and most often seen
ment of cutaneous T-cell lymphomas (CTCLs; see Chapter 85). after high-dose therapy.
CH 3 O O
CICH CH N CH CH CI CH S OCH CH CH CH O S CH
2 2 2 2 3 2 2 2 2 3
Mechlorethamine O Busulfan O
CONH 2
O N
CH
CICH CH 2 N CNHCH CH CI O 2
2
2
2
N N N
N O CNH 2 N CH 2
Carmustine [BCNU] Dacarbazine
N
N
N
N
N
CH 3
O
Temozolomide
Fig. 57.3 STRUCTURE OF COMMON ALKYLATING AGENTS.