Page 1630 - Williams Hematology ( PDFDrive )
P. 1630
1604 Part XI: Malignant Lymphoid Diseases Chapter 97: Hodgkin Lymphoma 1605
3.5 but not fraternal, twins provides the strongest evidence for a genetic
43
association. cHL-prone families, with or without other forms of
3
Incidence rate per 100,000 2.5 4.5 percent of cases are familial. 44–46 The standard incidence ratio for
cancer, have been described in the literature and it is estimated that
2
age-specific familial risk from the Swedish Cancer Registry was higher
1.5
47
for Hodgkin lymphoma (4.8) than any other neoplasm. The relative
risk for familial disease is stronger in individuals older than 40 years of
1
0.5
kemia and non-Hodgkin lymphoma has been described. An increased
46
0 age, males, and siblings, and a shared risk with chronic lymphocytic leu-
incidence in same sex siblings (eight- to 12-fold) versus opposite-sex
1975 1977 1979 1981 1983 1985 1987 1989 1991 1993 1995 1997 1999 2001 2003 2005 2007 2009 2011 siblings (1.3- to 1.4-fold) detected in the Swedish registry is consistent
Year with older data and has been interpreted to be supportive of an environ-
mental influence or a pseudoautosomal susceptibility gene located on a
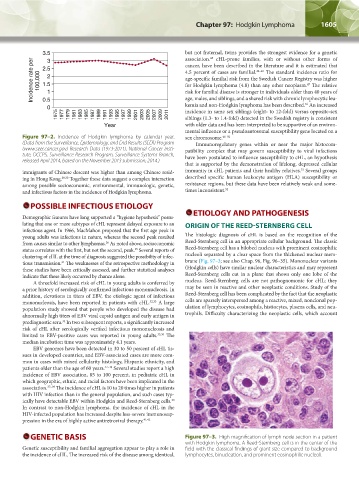
Figure 97–2. Incidence of Hodgkin lymphoma by calendar year. sex chromosome. 48–50
(Data from the Surveillance, Epidemiology, and End Results (SEER) Program Immunoregulatory genes within or near the major histocom-
(www.seer.cancer.gov) Research Data (1973-2011), National Cancer Insti- patibility complex that may govern susceptibility to viral infections
tute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, have been postulated to influence susceptibility to cHL, an hypothesis
released April 2014, based on the November 2013 submission; 2014.)
that is supported by the demonstration of lifelong, depressed cellular
51
immigrants of Chinese descent was higher than among Chinese resid- immunity in cHL patients and their healthy relatives. Several groups
ing in Hong Kong. 29,30 Together these data suggest a complex interaction described specific human leukocyte antigen (HLA) susceptibility or
among possible socioeconomic, environmental, immunologic, genetic, resistance regions, but these data have been relatively weak and some-
and infectious factors in the incidence of Hodgkin lymphoma. times inconsistent. 52
POSSIBLE INFECTIOUS ETIOLOGY
Demographic features have long supported a “hygiene hypothesis” postu- ETIOLOGY AND PATHOGENESIS
lating that one or more subtypes of cHL represent delayed exposure to an ORIGIN OF THE REED-STERNBERG CELL
infectious agent. In 1966, MacMahon proposed that the first age peak in
young adults was infectious in nature, whereas the second peak resulted The histologic diagnosis of cHL is based on the recognition of the
from causes similar to other lymphomas. As noted above, socioeconomic Reed-Sternberg cell in an appropriate cellular background. The classic
26
status correlates with the first, but not the second, peak. Several reports of Reed-Sternberg cell has a bilobed nucleus with prominent eosinophilic
25
clustering of cHL at the time of diagnosis suggested the possibility of infec- nucleoli separated by a clear space from the thickened nuclear mem-
tious transmission. The weaknesses of the retrospective methodology in brane (Fig. 97–3; see also Chap. 96, Fig. 96–35). Mononuclear variants
31
these studies have been critically assessed, and further statistical analyses (Hodgkin cells) have similar nuclear characteristics and may represent
indicate that these likely occurred by chance alone. Reed-Sternberg cells cut in a plane that shows only one lobe of the
A threefold increased risk of cHL in young adults is conferred by nucleus. Reed-Sternberg cells are not pathognomonic for cHL; they
a prior history of serologically confirmed infectious mononucleosis. In may be seen in reactive and other neoplastic conditions. Study of the
addition, elevations in titers of EBV, the etiologic agent of infectious Reed-Sternberg cell has been complicated by the fact that the neoplastic
mononucleosis, have been reported in patients with cHL. 32,33 A large cells are sparsely interspersed among a reactive, mixed, nonclonal pop-
population study showed that people who developed the disease had ulation of lymphocytes, eosinophils, histiocytes, plasma cells, and neu-
abnormally high titers of EBV viral capsid antigen and early antigen in trophils. Difficulty characterizing the neoplastic cells, which account
prediagnostic sera. In two subsequent reports, a significantly increased
34
risk of cHL after serologically verified infectious mononucleosis and
limited to EBV-positive cases was reported in young adults. 35,36 The
median incubation time was approximately 4.1 years.
EBV genomes have been detected in 30 to 50 percent of cHL tis-
sues in developed countries, and EBV-associated cases are more com-
mon in cases with mixed cellularity histology, Hispanic ethnicity, and
patients older than the age of 60 years. 37–39 Several studies report a high
incidence of EBV association, 85 to 100 percent, in pediatric cHL in
which geographic, ethnic, and racial factors have been implicated in the
association. 37–39 The incidence of cHL is 10 to 20 times higher in patients
with HIV infection than in the general population, and such cases typ-
ically have detectable EBV within Hodgkin and Reed-Sternberg cells.
40
In contrast to non-Hodgkin lymphoma, the incidence of cHL in the
HIV-infected population has increased despite less-severe immunosup-
pression in the era of highly active antiretroviral therapy. 41,42
GENETIC BASIS Figure 97–3. High magnification of lymph node section in a patient
with Hodgkin lymphoma. A Reed-Sternberg cell is in the center of the
Genetic susceptibility and familial aggregation appear to play a role in field with the classical findings of giant size compared to background
the incidence of cHL. The increased risk of the disease among identical, lymphocytes, binucleation, and prominent eosinophilic nucleoli.
Kaushansky_chapter 97_p1603-1624.indd 1605 9/18/15 11:11 PM