Page 1855 - Williams Hematology ( PDFDrive )
P. 1855
1830 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1831
OVERVIEW OF PLATELET ADHESION, platelet interactions with the blood vessel wall by forcing platelets to
the periphery of the bloodstream (as the erythrocytes disproportion-
AGGREGATION, AND PLATELET ately occupy the axial region), by imparting radially directed energy to
THROMBUS FORMATION platelets as the erythrocytes engage in flip-flop motions, and perhaps
by releasing the platelet activator adenosine diphosphate (ADP) at sites
2–4
The hemostatic system is under elaborate control mechanisms lest the of vascular injury ; and (5) the speed of blood flow and the size of the
response be either inadequate to meet the hemorrhagic challenge or blood vessel, which will determine the number of platelets passing by a
result in inappropriate thrombosis in response to trivial provocation. single point in a given time interval, the amount of time a platelet has
Evolutionary pressures have probably favored a more active hemostatic to interact with the blood vessel wall or other platelets, the rate of dilu-
system as individuals with more active hemostatic systems were more tion of platelet activating agents, and the forces tending to pull a platelet
likely to avoid death from hemorrhage prior to attaining sexual matu- from the vessel wall or another platelet (shear rate). 2,4–6 The vasospastic
rity or in association with childbirth. Our active hemostatic system may response that accompanies vascular injury, to which platelets contribute
be less-well adapted to our modern age, which is characterized by long by release of thromboxane (TX) A and serotonin, probably plays a key
2
life spans and progressive vascular disease, given that the deposition of role in decreasing hemorrhage and facilitating platelet and fibrin depo-
a platelet-fibrin thrombus on a damaged atherosclerotic plaque is the sition via its effect on blood flow.
cause of most myocardial infarctions and many strokes. The initial adhesion of platelets occurs to the adhesive proteins
The platelet’s major function is to seal openings in the vascular within the subendothelial layer immediately subjacent to the endo-
1,5
tree. It is appropriate, therefore, that the initiating signal for platelet thelium or to activated endothelium. The platelet expresses many
deposition and activation is exposure of underlying portions of the receptors that participate in adhesive interactions (Table 112–1).
blood vessel wall that are normally concealed from circulating platelets Intravital microscopy and ex vivo flow chamber studies indicate that
by an intact endothelial lining (Fig.112–1). Additional parameters that discoid platelets that show minimal or no evidence of activation can
1
probably control the platelet response are: (1) the depth of injury, with form the initial layers of platelet aggregates when laminar flow is dis-
deeper damage exposing more platelet-reactive materials and tissue rupted by a stenotic lesion, but that stable thrombus development
6
factor (Chap. 115); (2) the vascular bed, with the blood vessels serving requires the generation and/or release of soluble activators. Mem-
mucocutaneous tissues especially dependent on platelets for hemostasis, brane tethers, which can undergo restructuring and stabilization, are
in contrast to the vascular beds in muscles and joints, which rely more important in achieving interactions with matrix proteins and other
on the coagulation mechanism; (3) the age of the individual, because platelets.
the composition of the blood vessel wall probably changes with age; The shear rate differentially affects platelet adhesion to sur-
(4) the hematocrit, because increased numbers of erythrocytes enhance faces. 3,4,7–12 Shear rates, which reflect the differences in flow velocity
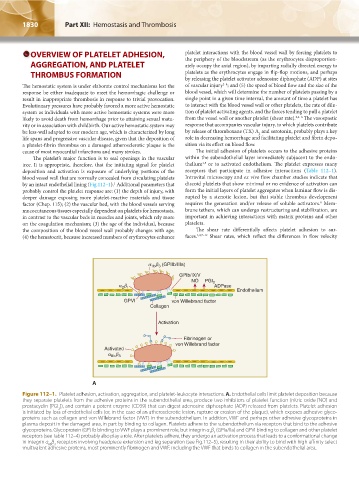
A
Figure 112–1. Platelet adhesion, activation, aggregation, and platelet-leukocyte interactions. A. Endothelial cells limit platelet deposition because
they separate platelets from the adhesive proteins in the subendothelial area, produce two inhibitors of platelet function (nitric oxide [NO] and
prostacyclin [PGI ]), and contain a potent enzyme (CD39) that can digest adenosine diphosphate (ADP) released from platelets. Platelet adhesion
2
is initiated by loss of endothelial cells (or, in the case of an atherosclerotic lesion, rupture or erosion of the plaque), which exposes adhesive glyco-
proteins such as collagen and von Willebrand factor (VWF) in the subendothelium. In addition, VWF and perhaps other adhesive glycoproteins in
plasma deposit in the damaged area, in part by binding to collagen. Platelets adhere to the subendothelium via receptors that bind to the adhesive
glycoproteins. Glycoprotein (GP) Ib binding to VWF plays a prominent role, but integrin α β (GPIa/IIa) and GPVI binding to collagen and other platelet
2 1
receptors (see Table 112–4) probably also play a role. After platelets adhere, they undergo an activation process that leads to a conformational change
in integrin α β receptors involving headpiece extension and leg separation (see Fig.112–5), resulting in their ability to bind with high-affinity select
IIb 3
multivalent adhesive proteins, most prominently fibrinogen and VWF, including the VWF that binds to collagen in the subendothelial area.
Kaushansky_chapter 112_p1829-1914.indd 1830 17/09/15 3:25 pm