Page 219 - Williams Hematology ( PDFDrive )
P. 219
194 Part IV: Molecular and Cellular Hematology Chapter 14: Metabolism of Hematologic Neoplastic Cells 195
mTORC2 PDK1 LKB
PTEN AMPK
growth
factor TSC1
RTK PI3K AKT TSC2 Rheb mTORC1
RAS RAF MEK RSK
ERK HIF-1α
MDM2
ribosome biogenesis CELL GROWTH
MYC protein synthesis
nutrients TP53 &
PROLIFERATION
glycolysis nucleic acid
lipid and
carbohydrate
glutaminolysis synthesis
wastes lactate
CO
2
NADPH
ROS
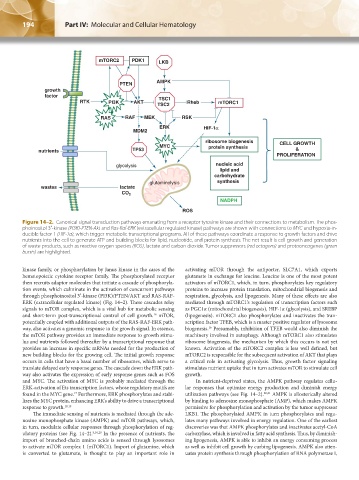
Figure 14–2. Canonical signal transduction pathways emanating from a receptor tyrosine kinase and their connections to metabolism. The phos-
phoinositol 3′-kinase (PI3K)-PTEN-Akt and Ras-Raf-ERK (extracellular regulated kinase) pathways are shown with connections to MYC and hypoxia-in-
ducible factor 1 (HIF-1α), which trigger metabolic transcriptional programs. All of these pathways coordinate a response to growth factors and drive
nutrients into the cell to generate ATP and building blocks for lipid, nucleotide, and protein synthesis. The net result is cell growth and generation
of waste products, such as reactive oxygen species (ROS), lactate and carbon dioxide. Tumor suppressors (red octagons) and protooncogenes (green
bursts) are highlighted.
kinase family, or phosphorylation by Janus kinase in the cases of the activating mTOR through the antiporter, SLC7A1, which exports
hematopoietic cytokine receptor family. The phosphorylated receptor glutamate in exchange for leucine. Leucine is one of the most potent
then recruits adaptor molecules that initiate a cascade of phosphoryla- activators of mTORC1, which, in turn, phosphorylates key regulatory
tion events, which culminate in the activation of concurrent pathways proteins to increase protein translation, mitochondrial biogenesis and
through phosphoinositol 3′-kinase (PI3K)/PTEN/AKT and RAS-RAF- respiration, glycolysis, and lipogenesis. Many of these effects are also
ERK (extracellular regulated kinase) (Fig. 14–2). These cascades relay mediated through mTORC1’s regulation of transcription factors such
signals to mTOR complex, which is a vital hub for metabolic sensing as PGC1α (mitochondrial biogenesis), HIF-1α (glycolysis), and SREBP
16
and short-term post-transcriptional control of cell growth. mTOR, (lipogenesis). mTORC1 also phosphorylates and inactivates the tran-
potentially coupled with additional outputs of the RAS-RAF-ERK path- scription factor TFEB, which is a master positive regulator of lysosome
way, also activates a genomic response to the growth signal. In essence, biogenesis. Presumably, inhibition of TFEB would also diminish the
16
the mTOR pathway provides an immediate response to growth stimu- machinery involved in autophagy. Although mTORC1 also stimulates
lus and nutrients followed thereafter by a transcriptional response that ribosome biogenesis, the mechanism by which this occurs is not yet
provides an increase in specific mRNAs needed for the production of known. Activation of the mTORC2 complex is less well defined, but
new building blocks for the growing cell. The initial growth response mTORC2 is responsible for the subsequent activation of AKT that plays
occurs in cells that have a basal number of ribosomes, which serve to a critical role in activating glycolysis. Thus, growth factor signaling
translate delayed early response genes. The cascade down the ERK path- stimulates nutrient uptake that in turn activates mTOR to stimulate cell
way also activates the expression of early response genes such as FOS growth.
and MYC. The activation of MYC is probably mediated through the In nutrient-deprived states, the AMPK pathway regulates cellu-
ERK-activation of Ets transcription factors, whose regulatory motifs are lar responses that optimize energy production and diminish energy
found in the MYC gene. Furthermore, ERK phosphorylates and stabi- utilization pathways (see Fig. 14–2). 20,21 AMPK is allosterically altered
17
lizes the MYC protein, enhancing ERK’s ability to drive a transcriptional by binding to adenosine monophosphate (AMP), which makes AMPK
response to growth. 18,19 permissive for phosphorylation and activation by the tumor suppressor
The immediate sensing of nutrients is mediated through the ade- LKB1. The phosphorylated AMPK in turn phosphorylates and regu-
nosine monophosphate kinase (AMPK) and mTOR pathways, which, lates many pathways involved in energy regulation. One of the earliest
in turn, modulate cellular responses through phosphorylation of reg- discoveries was that AMPK phosphorylates and inactivates acetyl-CoA
ulatory proteins (see Fig. 14–2). 9,16,20 In the presence of nutrients, the carboxylase, which is involved in fatty acid synthesis. Thus, by diminish-
import of branched-chain amino acids is sensed through lysosomes ing lipogenesis, AMPK is able to inhibit an energy consuming process
to activate mTOR complex 1 (mTORC1). Import of glutamine, which as well as inhibit cell growth by curbing lipogenesis. AMPK also atten-
is converted to glutamate, is thought to play an important role in uates protein synthesis through phosphorylation of RNA polymerase I,
Kaushansky_chapter 14_p0191-0202.indd 194 17/09/15 6:35 pm