Page 319 - Williams Hematology ( PDFDrive )
P. 319
294 Part IV: Molecular and Cellular Hematology Chapter 20: Innate Immunity 295
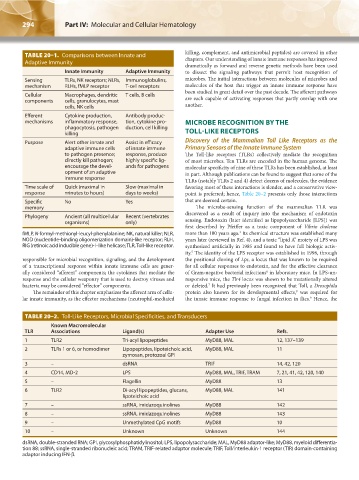
TABLE 20–1. Comparisons between Innate and killing, complement, and antimicrobial peptides) are covered in other
chapters. Our understanding of innate immune responses has improved
Adaptive Immunity
dramatically as forward and reverse genetic methods have been used
Innate Immunity Adaptive Immunity to dissect the signaling pathways that permit host recognition of
Sensing TLRs, NK receptors; NLRs, Immunoglobulins, microbes. The initial interactions between molecules of microbes and
mechanism RLHs, fMLP receptor T-cell receptors molecules of the host that trigger an innate immune response have
been studied in great detail over the past decade. The afferent pathways
Cellular Macrophages, dendritic T cells, B cells
components cells, granulocytes, mast are each capable of activating responses that partly overlap with one
cells, NK cells another.
Efferent Cytokine production, Antibody produc-
mechanisms inflammatory response, tion, cytokine pro- MICROBE RECOGNITION BY THE
phagocytosis, pathogen duction, cell killing
killing TOLL-LIKE RECEPTORS
Purpose Alert other innate and Assist in efficacy Discovery of the Mammalian Toll Like Receptors as the
adaptive immune cells of innate immune Primary Sensors of the Innate Immune System
to pathogen presence; response, produce The Toll-like receptors (TLRs) collectively mediate the recognition
directly kill pathogen; highly specific lig- of most microbes. Ten TLRs are encoded in the human genome. The
encourage the devel- ands for pathogens molecular specificity of nine of these TLRs has been established, at least
opment of an adaptive in part. Although publications can be found to suggest that some of the
immune response
TLRs (notably TLRs 2 and 4) detect dozens of molecules, the evidence
Time scale of Quick (maximal in Slow (maximal in favoring most of these interactions is slender, and a conservative view-
response minutes to hours) days to weeks) point is preferred; hence, Table 20–2 presents only those interactions
Specific No Yes that are deemed certain.
memory The microbe-sensing function of the mammalian TLR was
discovered as a result of inquiry into the mechanism of endotoxin
Phylogeny Ancient (all multicellular Recent (vertebrates
organisms) only) sensing. Endotoxin (later identified as lipopolysaccharide [LPS]) was
first described by Pfeiffer as a toxic component of Vibrio cholerae
3
fMLP, N-formyl-methionyl-leucyl-phenylalanine; NK, natural killer; NLR, more than 100 years ago. Its chemical structure was established many
NOD (nucleotide-binding oligomerization domain)-like receptor; RLH, years later (reviewed in Ref. 4), and a toxic “lipid A” moiety of LPS was
RIG (retinoic acid inducible gene)-I–like helicase; TLR, Toll-like receptor. synthesized artificially in 1985 and found to have full biologic activ-
5
ity. The identity of the LPS receptor was established in 1998, through
responsible for microbial recognition, signaling, and the development the positional cloning of Lps, a locus that was known to be required
of a transcriptional response within innate immune cells are gener- for all cellular responses to endotoxin, and for the effective clearance
6
ally considered “afferent” components; the cytokines that mediate the of Gram-negative bacterial infections in laboratory mice. In LPS-un-
response and the cellular weaponry that is used to destroy viruses and responsive mice, the Tlr4 locus was shown to be mutationally altered
bacteria may be considered “effector” components. or deleted. It had previously been recognized that Toll, a Drosophila
7
The remainder of this chapter emphasizes the afferent arm of cellu- protein also known for its developmental effects, was required for
8
9
lar innate immunity, as the effector mechanisms (neutrophil-mediated the innate immune response to fungal infection in flies. Hence, the
TABLE 20–2. Toll-Like Receptors, Microbial Specificities, and Transducers
Known Macromolecular
TLR Associations Ligand(s) Adapter Use Refs.
1 TLR2 Tri-acyl lipopeptides MyD88, MAL 12, 137–139
2 TLRs 1 or 6, or homodimer Lipopeptides, lipoteichoic acid, MyD88, MAL 11
zymosan, protozoal GPI
3 – dsRNA TRIF 14, 42, 120
4 CD14, MD-2 LPS MyD88, MAL, TRIF, TRAM 7, 21, 41, 42, 120, 140
5 – Flagellin MyD88 13
6 TLR2 Di-acyl lipopeptides, glucans, MyD88, MAL 141
lipoteichoic acid
7 – ssRNA, imidazoquinolines MyD88 142
8 – ssRNA, imidazoquinolines MyD88 143
9 – Unmethylated CpG motifs MyD88 10
10 – Unknown Unknown 144
dsRNA, double-stranded RNA; GPI, glycosylphosphatidylinositol; LPS, lipopolysaccharide; MAL, MyD88 adaptor-like; MyD88, myeloid differentia-
tion 88; ssRNA, single-stranded ribonucleic acid; TRAM, TRIF-related adaptor molecule; TRIF, Toll/interleukin-1 receptor (TIR) domain-containing
adaptor inducing IFN-β.
Kaushansky_chapter 20_p0293-0306.indd 294 9/17/15 5:51 PM