Page 324 - Williams Hematology ( PDFDrive )
P. 324
298 Part IV: Molecular and Cellular Hematology Chapter 20: Innate Immunity 299
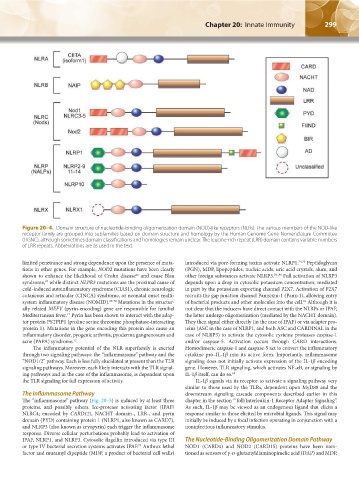
Figure 20–4. Domain structure of nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). The various members of the NOD-like
receptor family are grouped into subfamilies based on domain structure and homology by the Human Genome Gene Nomenclature Committee
(HGNC), although sometimes domain classifications and homologies remain unclear. The leucine-rich repeat (LRR) domain contains variable numbers
of LRR repeats. Abbreviations are as used in the text.
limited penetrance and strong dependence upon the presence of muta- introduced via pore-forming toxins activate NLRP1. 74,75 Peptidoglycan
tions in other genes. For example, NOD2 mutations have been clearly (PGN), MDP, lipopeptides, nucleic acids, uric acid crystals, alum, and
shown to enhance the likelihood of Crohn disease and cause Blau other foreign substances activate NLRP3. 76–80 Full activation of NLRP3
66
syndrome, while distinct NLPR3 mutations are the proximal cause of depends upon a drop in cytosolic potassium concentration, mediated
67
cold-induced autoinflammatory syndrome (CIAS1), chronic neurologic in part by the potassium exporting channel P2X7. Activation of P2X7
cutaneous and articular (CINCA) syndrome, or neonatal onset multi- recruits the gap junction channel Pannexin-1 (Panx-1), allowing entry
system inflammatory disease (NOMID). 68-70 Mutations in the structur- of bacterial products and other molecules into the cell. Although it is
81
ally related MEFV (pyrin-encoding) gene are responsible for familial not clear that the inducers have direct contact with the NLRPs or IPAF,
Mediterranean fever. Pyrin has been shown to interact with the adap- the latter undergo oligomerization (mediated by the NACHT domain).
71
tor protein PSTPIP1 (proline serine threonine phosphatase-interacting They then signal either directly (in the case of IPAF) or via adapter pro-
protein 1). Mutations in the gene encoding this protein also cause an teins (ASC in the case of NLRP1, and both ASC and CARDINAL in the
inflammatory disorder, pyogenic arthritis, pyoderma gangrenosum and case of NLRP3) to activate the cytosolic cysteine proteases caspase-1
acne (PAPA) syndrome. 72 and/or caspase-5. Activation occurs through CARD interactions.
The inflammatory potential of the NLR superfamily is exerted Homodimeric caspase-1 and caspase-5 act to convert the inflammatory
through two signaling pathways: the “inflammasome” pathway and the cytokine pro–IL-1β into its active form. Importantly, inflammasome
“NOD1/2” pathway. Each is less fully elucidated at present than the TLR signaling does not initially activate expression of the IL-1β encoding
signaling pathways. Moreover, each likely interacts with the TLR signal- gene. However, TLR signaling, which activates NF-κB, or signaling by
ing pathways and in the case of the inflammasome, is dependent upon IL-1β itself, can do so. 65
the TLR signaling for full expression of activity. IL-1β signals via its receptor to activate a signaling pathway very
similar to those used by the TLRs, dependent upon MyD88 and the
The Inflammasome Pathway downstream signaling cascade components described earlier in this
The “inflammasome” pathway (Fig. 20–5) is induced by at least three chapter in the section “Toll/Interleukin-1 Receptor Adapter Signaling”.
proteins, and possibly others. Ice-protease activating factor (IPAF/ As such, IL-1β may be viewed as an endogenous ligand that elicits a
NLRC4; encoded by CARD12), NACHT domain-, LRR-, and pyrin response similar to those elicited by microbial ligands. This signal may
domain (PYD) containing protein 1 (NLRP1, also known as CARD7), initially be induced by a focal infection operating in conjunction with a
and NLRP3 (also known as cryopyrin) each trigger the inflammasome noninfectious inflammatory stimulus.
response. Diverse cellular perturbations probably lead to activation of
IPAF, NLRP1, and NLRP3. Cytosolic flagellin introduced via type III The Nucleotide-Binding Oligomerization Domain Pathway
or type IV bacterial secretion systems activates IPAF. Anthrax lethal NOD1 (CARD4) and NOD2 (CARD15) proteins have been men-
73
factor and muramyl dipeptide (MDP, a product of bacterial cell walls) tioned as sensors of γ-d-glutamyldiaminopimelic acid (DAP) and MDP,
Kaushansky_chapter 20_p0293-0306.indd 299 9/17/15 5:52 PM