Page 717 - Williams Hematology ( PDFDrive )
P. 717
692 Part VI: The Erythrocyte Chapter 47: Erythrocyte Enzyme Disorders 693
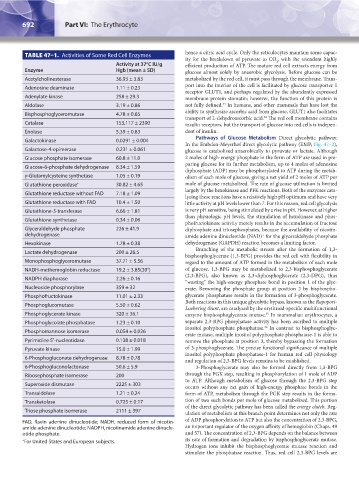
TABLE 47–1. Activities of Some Red Cell Enzymes hence a citric acid cycle. Only the reticulocytes maintain some capac-
ity for the breakdown of pyruvate to CO , with the attendant highly
2
Activity at 37°C IU/g efficient production of ATP. The mature red cell extracts energy from
Enzyme Hgb (mean ± SD) glucose almost solely by anaerobic glycolysis. Before glucose can be
Acetylcholinesterase 36.93 ± 3.83 metabolized by the red cell, it must pass through the membrane. Trans-
Adenosine deaminase 1.11 ± 0.23 port into the interior of the cell is facilitated by glucose transporter 1
receptor GLUT1, and perhaps regulated by the abundantly expressed
Adenylate kinase 258 ± 29.3 membrane protein stomatin; however, the function of this protein is
34
Aldolase 3.19 ± 0.86 not fully defined. In humans, and other mammals that have lost the
Bisphosphoglyceromutase 4.78 ± 0.65 ability to synthesize ascorbic acid from glucose, GLUT1 also facilitates
transport of L-dehydroascorbic acid. The red cell membrane contains
34
Catalase 153,117 ± 2390 insulin receptors, but the transport of glucose into red cells is indepen-
Enolase 5.39 ± 0.83 dent of insulin.
Pathways of Glucose Metabolism Direct glycolytic pathway.
Galactokinase 0.0291 ± 0.004
In the Embden-Meyerhof direct glycolytic pathway (EMP; Fig. 47–2),
Galactose-4-epimerase 0.231 ± 0.061 glucose is catabolized anaerobically to pyruvate or lactate. Although
Glucose phosphate isomerase 60.8 ± 11.0 2 moles of high-energy phosphate in the form of ATP are used in pre-
paring glucose for its further metabolism, up to 4 moles of adenosine
Glucose-6-phosphate dehydrogenase 8.34 ± 1.59
diphosphate (ADP) may be phosphorylated to ATP during the metab-
γ-Glutamylcysteine synthetase 1.05 ± 0.19 olism of each mole of glucose, giving a net yield of 2 moles of ATP per
Glutathione peroxidase * 30.82 ± 4.65 mole of glucose metabolized. The rate of glucose utilization is limited
largely by the hexokinase and PFK reactions. Both of the enzymes cata-
Glutathione reductase without FAD 7.18 ± 1.09
lyzing these reactions have a relatively high pH optimum and have very
Glutathione reductase with FAD 10.4 ± 1.50 little activity at pH levels lower than 7. For this reason, red cell glycolysis
Glutathione-S-transferase 6.66 ± 1.81 is very pH sensitive, being stimulated by a rise in pH. However, at higher
than physiologic pH levels, the stimulation of hexokinase and phos-
Glutathione synthetase 0.34 ± 0.06
phofructokinase activity merely results in the accumulation of fructose
Glyceraldehyde phosphate 226 ± 41.9 diphosphate and triosephosphates, because the availability of nicotin-
dehydrogenase amide adenine dinucleotide (NAD) for the glyceraldehyde phosphate
+
Hexokinase 1.78 ± 0.38 dehydrogenase (GAPDH) reaction becomes a limiting factor.
Branching of the metabolic stream after the formation of 1,3-
Lactate dehydrogenase 200 ± 26.5
bisphosphoglycerate (1,3-BPG) provides the red cell with flexibility in
Monophosphoglyceromutase 37.71 ± 5.56 regard to the amount of ATP formed in the metabolism of each mole
NADH-methemoglobin reductase 19.2 ± 3.85(30°) of glucose. 1,3-BPG may be metabolized to 2,3-bisphosphoglycerate
(2,3-BPG), also known as 2,3-diphosphoglycerate (2,3-DPG), thus
NADPH diaphorase 2.26 ± 0.16
“wasting” the high-energy phosphate bond in position 1 of the glyc-
Nucleoside phosphorylase 359 ± 32 erate. Removing the phosphate group at position 2 by bisphospho-
Phosphofructokinase 11.01 ± 2.33 glycerate phosphatase results in the formation of 3-phosphoglycerate.
Both reactions in this unique glycolytic bypass, known as the Rapoport-
Phosphoglucomutase 5.50 ± 0.62
Luebering shunt, are catalyzed by the erythroid-specific multifunctional
Phosphoglycerate kinase 320 ± 36.1 enzyme bisphosphoglycerate mutase. In mammalian erythrocytes, a
35
Phosphoglycolate phosphatase 1.23 ± 0.10 separate 2,3-BPG phosphatase activity has been ascribed to multiple
36
inositol polyphosphate phosphatase. In contrast to bisphosphoglyc-
Phosphomannose isomerase 0.054 ± 0.026
erate mutase, multiple inositol polyphosphate phosphatase-1 is able to
Pyrimidine 5′-nucleotidase 0.138 ± 0.018 remove the phosphate at position 3, thereby bypassing the formation
Pyruvate kinase 15.0 ± 1.99 of 3-phosphoglycerate. The precise functional significance of multiple
inositol polyphosphate phosphatase-1 for human red cell physiology
6-Phosphogluconate dehydrogenase 8.78 ± 0.78
and regulation of 2,3-BPG levels remains to be established.
6-Phosphogluconolactonase 50.6 ± 5.9 3-Phosphoglycerate may also be formed directly from 1,3-BPG
Ribosephosphate isomerase 200 through the PGK step, resulting in phosphorylation of 1 mole of ADP
to ATP. Although metabolism of glucose through the 2,3-BPG step
Superoxide dismutase 2225 ± 303
occurs without any net gain of high-energy phosphate bonds in the
Transaldolase 1.21 ± 0.24 form of ATP, metabolism through the PGK step results in the forma-
Transketolase 0.725 ± 0.17 tion of two such bonds per mole of glucose metabolized. This portion
of the direct glycolytic pathway has been called the energy clutch. Reg-
Triose phosphate isomerase 2111 ± 397
ulation of metabolism at this branch point determines not only the rate
FAD, flavin adenine dinucleotide; NADH, reduced form of nicotin- of ADP phosphorylation to ATP but also the concentration of 2,3-BPG,
amide adenine dinucleotide; NADPH, nicotinamide adenine dinucle- an important regulator of the oxygen affinity of hemoglobin (Chaps. 49
otide phosphate. and 57). The concentration of 2,3-BPG depends on the balance between
* For United States and European subjects. its rate of formation and degradation by bisphosphoglycerate mutase.
Hydrogen ions inhibit the bisphosphoglycerate mutase reaction and
stimulate the phosphatase reaction. Thus, red cell 2,3-BPG levels are
Kaushansky_chapter 47_p0689-0724.indd 692 9/17/15 6:44 PM