Page 881 - Williams Hematology ( PDFDrive )
P. 881
856 Part VI: The Erythrocyte Chapter 55: Alloimmune Hemolytic Disease of the Fetus and Newborn 857
A B
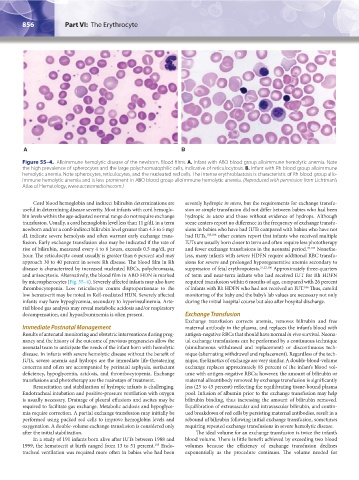
Figure 55–4. Alloimmune hemolytic disease of the newborn. Blood films. A. Infant with ABO blood group alloimmune hemolytic anemia. Note
the high prevalence of spherocytes and the large polychromatophilic cells, indicative of reticulocytosis. B. Infant with Rh blood group alloimmune
hemolytic anemia. Note spherocytes, reticulocytes, and the nucleated red cells. The intense erythroblastosis is characteristic of Rh blood group allo-
immune hemolytic anemia and is less prominent in ABO blood group alloimmune hemolytic anemia. (Reproduced with permission from Lichtman’s
Atlas of Hematology, www.accessmedicine.com.)
Cord blood hemoglobin and indirect bilirubin determinations are severely hydropic in utero, but the requirements for exchange transfu-
useful in determining disease severity. Most infants with cord hemoglo- sion or simple transfusion did not differ between babies who had been
bin levels within the age-adjusted normal range do not require exchange hydropic in utero and those without evidence of hydrops. Although
transfusion. Usually, a cord hemoglobin level less than 11 g/dL in a term some centers report no difference in the frequency of exchange transfu-
newborn and/or a cord-indirect bilirubin level greater than 4.5 to 5 mg/ sions in babies who have had IUTs compared with babies who have not
dL indicate severe hemolysis and often warrant early exchange trans- had IUTs, 105,106 other centers report that infants who received multiple
fusion. Early exchange transfusion also may be indicated if the rate of IUTs are usually born closer to term and often require less phototherapy
rise of bilirubin, measured every 4 to 6 hours, exceeds 0.5 mg/dL per and fewer exchange transfusions in the neonatal period. 25,107 Nonethe-
hour. The reticulocyte count usually is greater than 6 percent and may less, many infants with severe HDFN require additional RBC transfu-
approach 30 to 40 percent in severe Rh disease. The blood film in Rh sions for severe and prolonged hyporegenerative anemia secondary to
disease is characterized by increased nucleated RBCs, polychromasia, suppression of fetal erythropoiesis. 21,22,106 Approximately three-quarters
and anisocytosis. Alternatively, the blood film in ABO HDN is marked of term and near-term infants who had received IUT for Rh HDFN
by microspherocytes (Fig. 55–4). Severely affected infants may also have required transfusion within 6 months of age, compared with 26 percent
thrombocytopenia. Low reticulocyte counts disproportionate to the of infants with Rh HDFN who had not received an IUT. Thus, careful
106
low hematocrit may be noted in Kell-mediated HDN. Severely affected monitoring of the baby and the baby’s lab values are necessary not only
infants may have hypoglycemia, secondary to hyperinsulinemia. Arte- during the initial hospital course but also after hospital discharge.
rial blood gas analysis may reveal metabolic acidosis and/or respiratory
decompensation, and hypoalbuminemia is often present. Exchange Transfusion
Exchange transfusion corrects anemia, removes bilirubin and free
Immediate Postnatal Management maternal antibody in the plasma, and replaces the infant’s blood with
Results of antenatal monitoring and obstetric interventions during preg- antigen-negative RBCs that should have normal in vivo survival. Neona-
nancy and the history of the outcome of previous pregnancies allow the tal exchange transfusions can be performed by a continuous technique
neonatal team to anticipate the needs of the infant born with hemolytic (simultaneous withdrawal and replacement) or discontinuous tech-
disease. In infants with severe hemolytic disease without the benefit of nique (alternating withdrawal and replacement). Regardless of the tech-
IUTs, severe anemia and hydrops are the immediate life-threatening nique, the kinetics of exchange are very similar. A double-blood-volume
concerns and often are accompanied by perinatal asphyxia, surfactant exchange replaces approximately 85 percent of the infant’s blood vol-
deficiency, hypoglycemia, acidosis, and thrombocytopenia. Exchange ume with antigen-negative RBCs; however, the amount of bilirubin or
transfusions and phototherapy are the mainstays of treatment. maternal alloantibody removed by exchange transfusion is significantly
Resuscitation and stabilization of hydropic infants is challenging. less (25 to 45 percent) reflecting the equilibrating tissue-bound plasma
Endotracheal intubation and positive-pressure ventilation with oxygen pool. Infusion of albumin prior to the exchange transfusion may help
is usually necessary. Drainage of pleural effusions and ascites may be bilirubin binding, thus increasing the amount of bilirubin removed.
required to facilitate gas exchange. Metabolic acidosis and hypoglyce- Equilibration of extravascular and intravascular bilirubin, and contin-
mia require correction. A partial exchange transfusion may initially be ued breakdown of red cells by persisting maternal antibodies, result in a
performed using packed red cells to improve hemoglobin levels and rebound of bilirubin following initial exchange transfusion, sometimes
oxygenation. A double-volume exchange transfusion is considered only requiring repeated exchange transfusions in severe hemolytic disease.
after the initial stabilization. The ideal volume for an exchange transfusion is twice the infant’s
In a study of 191 infants born alive after IUTs between 1988 and blood volume. There is little benefit achieved by exceeding two blood
1999, the hematocrit at birth ranged from 13 to 51 percent. Endo- volumes because the efficiency of exchange transfusion declines
105
tracheal ventilation was required more often in babies who had been exponentially as the procedure continues. The volume needed for
Kaushansky_chapter 55_p0847-0862.indd 856 9/18/15 11:52 PM