Page 95 - Williams Hematology ( PDFDrive )
P. 95
70 Part II: The Organization of the Lymphohematopoietic Tissues Chapter 5: Structure of the Marrow and the Hematopoietic Microenvironment 71
In the marrow, multiple mechanisms act to stabilize and reinforce they penetrate the cytoplasm of the endothelial cell and enter the sinus
391
the lodgment of HSC, that is, to maintain the HSC in niches. One prom- lumen (Fig. 5–7). As with reticulocytes, egress occurs adjacent to
383
inent mechanism is the binding of SCF, either secreted in and adherent junctions of endothelial cells. The nucleus of the granulocyte, usually
to the marrow matrix or displayed on stromal cells. The absence of either segmented, does not require as marked a deformation to traverse the
391
KIT or SCF results in embryonic failure of hematopoiesis as a result of migration pore as do the nuclei of monocytes and lymphocytes. This
impaired homing of HSCs to the fetal liver where SCF acts coopera- transendothelial migration is likely to be related to leukocyte migration
tively with CXCL12 as a chemoattractant, and to impaired retention of from the blood and into areas of inflammation described in the section
513
HSCs in the marrow where KIT upregulates HSC expression of inte- on adhesion and homing because the marrow sinusoidal endothelial
514
grins α β and α β . The β integrins of the HSCs also bind osteopon- cells constitutively express adhesion proteins that are upregulated in
4 1
5 1
1
tin, which, in turn, is bound to other matrix proteins, such as FN and inflammation, including VCAM-1, ICAM-1, and E- and P-selectin.
405
collagen. Similarly, CD44 on HSCs binds to hyaluronic acid, FN, and Immature granulocytes in the marrow are anchored to adventitial
collagen the marrow matrix. Two receptors on HSCs that contribute reticular cells through lectin-like adhesion molecules. Gradual loss of
164
specifically to endosteal niche retention are the calcium-sensing recep- these molecules (e.g., shedding of L-selectin) during maturation or after
tor, which is needed for effective binding to collagen, and the Tie fam- activation could permit movement toward the sinus wall. Transient
521
515
ily receptor kinases, specifically Tie-2 receptor, which mediates HSC changes in surface glycoproteins (upregulation of α-2,6-sialylation of
integrin binding to FN after engaging its ligand, angiopoitein-1, that CD11b and CD18) of maturing marrow myeloid cells lead to decreased
is expressed by osteoblasts. 516,517 Marrow SP cells enriched with long- stromal and FN adhesion and may favor contact with endothelium and
term repopulating quiescent HSCs display high expression of β -inte- cell egress. The complement component C5a and G-CSF administra-
522
3
grin, most likely as the vitronectin receptor α β , suggesting another tion recruit neutrophils by altering integrins (low CD11a with G-CSF)
v 3
integrin–matrix protein interaction that supports HSC retention. 445,518 and decreased L-selectin expression (with both agents). 523,524 Similar
One mechanism of retention in the endosteal niche is the long-term findings obtained in mice lacking two or all three selectins underscore
maintenance of HSCs by TPO produced by adjacent osteoblasts. 519,520 the essential role of selectins in neutrophil recruitment. Mature leu-
525
The binding of TPO by its receptor induces HSC quiescence, whereas kocytes retain their nuclei as they enter the marrow venous sinuses and
the absence of TPO leads to active cell cycling and to a protracted and circulate in the blood, but erythroid and megakaryocytic cells release
progressive depletion of HSCs. 519,520 anucleate cells and their residual nuclei are rapidly phagocytosed by
marrow macrophages. 94,384,526 Occasional immature granulocytes and
CELLULAR RELEASE megakaryocyte nuclei or whole megakaryocytes are present in cell con-
527
centrates of normal blood. Restrictions on the release of immature
Cell migration from the marrow occurs between adventitial cells and myeloid cells, erythroblasts, and megakaryocytes are associated with the
through endothelial cell channels that develop at the time of cell tran- relative stiffness of their nuclei because of the ratio of nuclear lamin
sit. Electron micrographs of leukocytes partially translocated across isotypes in erythroid and immature myeloid precursors and increased
endothelium indicate that marked deformation of these cells occurs as total lamins in megakaryocytes. 380
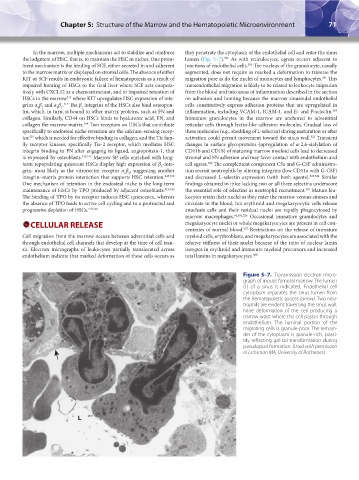
Figure 5–7. Transmission electron micro-
graph of mouse femoral marrow. The lumen
(L) of a sinus is indicated. Endothelial cell
cytoplasm separates the sinus lumen from
the hematopoietic spaces (arrow). Two neu-
trophils are evident traversing the sinus wall.
Note deformation of the cell producing a
narrow waist where the cell passes through
endothelium. The luminal portion of the
migrating cells is granule-poor. The remain-
der of the cytoplasm is granule-rich, possi-
bly reflecting gel-sol transformation during
pseudopod formation. (Used with permission
of Lichtman MA, University of Rochester.)
L
Kaushansky_chapter 05_p0051-0084.indd 71 9/19/15 12:11 AM